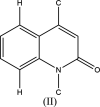
Crystal structure, Hirshfeld surface analysis, inter-action energy and energy framework calculations, as well as density functional theory (DFT) com-putation, of methyl 2-oxo-1-(prop-2-yn-yl)-1,2-di-hydro-quinoline-4-carboxyl-ate
- PMID: 37817963
- PMCID: PMC10561202
- DOI: 10.1107/S2056989023007557
Crystal structure, Hirshfeld surface analysis, inter-action energy and energy framework calculations, as well as density functional theory (DFT) com-putation, of methyl 2-oxo-1-(prop-2-yn-yl)-1,2-di-hydro-quinoline-4-carboxyl-ate
Abstract
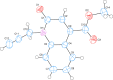
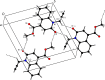
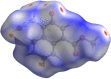
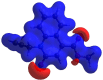
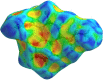
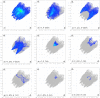
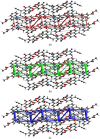
In the title mol-ecule, C14H11NO3, the di-hydro-quinoline core deviates slightly from planarity, indicated by the dihedral angle of 1.07 (3)° between the two six-membered rings. In the crystal, layers of mol-ecules almost parallel to the bc plane are formed by C-H⋯O hydro-gen bonds. These are joined by π-π stacking inter-actions. A Hirshfeld surface analysis revealed that the most important contributions to the crystal packing are from H⋯H (36.0%), H⋯C/C⋯H (28.9%) and H⋯O/O⋯H (23.5%) inter-actions. The evaluation of the electrostatic, dispersion and total energy frameworks indicates that the stabilization is dominated by the dispersion energy contribution. Moreover, the mol-ecular structure optimized by density functional theory (DFT) at the B3LYP/6-311G(d,p) level is com-pared with the experimentally determined mol-ecular structure in the solid state. The HOMO-LUMO behaviour was elucidated to determine the energy gap.
Keywords: C—H⋯O hydrogen bonds; crystal structure; dihydroquinoline; π-stacking.
© El-Mrabet et al. 2023.
Figures


Similar articles
-
Crystal structure, Hirshfeld surface analysis, inter-action energy and DFT calculations and energy frameworks of methyl 6-chloro-1-methyl-2-oxo-1,2-di-hydro-quinoline-4-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2022 Mar 22;78(Pt 4):425-432. doi: 10.1107/S2056989022002912. eCollection 2022 Apr 1. Acta Crystallogr E Crystallogr Commun. 2022. PMID: 35492275 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and inter-action energy and DFT studies of 2-chloro-ethyl 2-oxo-1-(prop-2-yn-1-yl)-1,2-di-hydro-quinoline-4-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2019 Sep 6;75(Pt 10):1411-1417. doi: 10.1107/S2056989019012283. eCollection 2019 Oct 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31636967 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and inter-action energy and DFT studies of 1-methyl-3-(prop-2-yn-1-yl)-2,3-di-hydro-1H-1,3-benzo-diazol-2-one.Acta Crystallogr E Crystallogr Commun. 2019 Nov 29;75(Pt 12):1940-1946. doi: 10.1107/S2056989019015779. eCollection 2019 Dec 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31871762 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and DFT studies of ethyl 2-{4-[(2-eth-oxy-2-oxoeth-yl)(phen-yl)carbamo-yl]-2-oxo-1,2-di-hydro-quinolin-1-yl}acetate.Acta Crystallogr E Crystallogr Commun. 2019 Oct 29;75(Pt 11):1753-1758. doi: 10.1107/S2056989019014154. eCollection 2019 Nov 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31709103 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and density functional theory study of benzyl 2-oxo-1-(prop-2-yn-1-yl)-1,2-di-hydro-quinoline-4-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2021 Jul 23;77(Pt 8):824-828. doi: 10.1107/S2056989021007416. eCollection 2021 Aug 1. Acta Crystallogr E Crystallogr Commun. 2021. PMID: 34422309 Free PMC article.
Cited by
-
Crystal structure and Hirshfeld surface analyses, inter-action energy calculations and energy frameworks of methyl 2-[(4-cyano-phen-yl)meth-oxy]quinoline-4-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2025 Jun 27;81(Pt 7):650-656. doi: 10.1107/S2056989025005547. eCollection 2025 Jul 1. Acta Crystallogr E Crystallogr Commun. 2025. PMID: 40630666 Free PMC article.
References
-
- Abdel-Wahab, B. F., Khidre, R. E., Farahat, A. A. & El-Ahl, A. S. (2012). Arkivoc, pp. 211–276.
-
- Baba, Y. F., Gökce, H., Rodi, Y. K., Hayani, S., Chahdi, F. O., Boukir, A., Jasinski, J. P., Kaur, M., Hökelek, T., Sebbar, N. K. & Essassi, E. M. (2020). J. Mol. Struct. 1217, 128461.
-
- Becke, A. D. (1993). J. Chem. Phys. 98, 5648–5652.
-
- Bouzian, Y., Hlimi, F., Sebbar, N. K., El Hafi, M., Hni, B., Essassi, E. M. & Mague, J. T. (2018). IUCrData, 3, x181438.
-
- Bruker (2019). APEX3 and SAINT, Bruker AXS, Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
