Engineering NK-CAR.19 cells with the IL-15/IL-15Rα complex improved proliferation and anti-tumor effect in vivo
- PMID: 37818365
- PMCID: PMC10561086
- DOI: 10.3389/fimmu.2023.1226518
Engineering NK-CAR.19 cells with the IL-15/IL-15Rα complex improved proliferation and anti-tumor effect in vivo
Abstract
Introduction: Natural killer 92 (NK-92) cells are an attractive therapeutic approach as alternative chimeric antigen receptor (CAR) carriers, different from T cells, once they can be used in the allogeneic setting. The modest in vivo outcomes observed with NK-92 cells continue to present hurdles in successfully translating NK-92 cell therapies into clinical applications. Adoptive transfer of CAR-NK-92 cells holds out the promise of therapeutic benefit at a lower rate of adverse events due to the absence of GvHD and cytokine release syndrome. However, it has not achieved breakthrough clinical results yet, and further improvement of CAR-NK-92 cells is necessary.
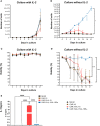
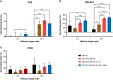
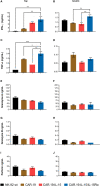
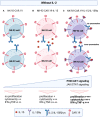
Methods: In this study, we conducted a comparative analysis between CD19-targeted CAR (CAR.19) co-expressing IL-15 (CAR.19-IL15) with IL-15/IL-15Rα (CAR.19-IL15/IL15Rα) to promote NK cell proliferation, activation, and cytotoxic activity against B-cell leukemia. CAR constructs were cloned into lentiviral vector and transduced into NK-92 cell line. Potency of CAR-NK cells were assessed against CD19-expressing cell lines NALM-6 or Raji in vitro and in vivo in a murine model. Tumor burden was measured by bioluminescence.
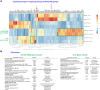
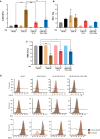
Results: We demonstrated that a fourth- generation CD19-targeted CAR (CAR.19) co-expressing IL-15 linked to its receptor IL-15/IL-15Rα (CAR.19-IL-15/IL-15Rα) significantly enhanced NK-92 cell proliferation, proinflammatory cytokine secretion, and cytotoxic activity against B-cell cancer cell lines in vitro and in a xenograft mouse model.
Conclusion: Together with the results of the systematic analysis of the transcriptome of activated NK-92 CAR variants, this supports the notion that IL-15/IL-15Rα comprising fourth-generation CARs may overcome the limitations of NK-92 cell-based targeted tumor therapies in vivo by providing the necessary growth and activation signals.
Keywords: B-cell malignances; CAR-NK cells; IL-15; IL-15 receptor; NK-92; adoptive cell therapy; allogeneic therapy.
Copyright © 2023 Silvestre, Eitler, de Azevedo, Tirapelle, Fantacini, de Souza, Swiech, Covas, Calado, Montero, Malmegrim, Figueiredo, Tonn and Picanço-Castro.
Conflict of interest statement
VPC, DTC, RNS, RC and JTCA are inventors of the patent PCT/BR2023/050127 owned by their respective institutions. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AT declared a past co-authorship with the author TT, and declared a shared affiliation with several of the authors JE, PM, TT to the handling editor at the time of the review.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

