Immunohistochemical Study of Airways Fibrous Remodeling in Smoking Mice
- PMID: 37818941
- PMCID: PMC10617442
- DOI: 10.1369/00221554231204926
Immunohistochemical Study of Airways Fibrous Remodeling in Smoking Mice
Abstract
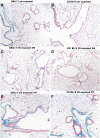
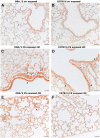
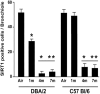
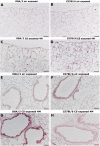
The fibrotic remodeling in chronic obstructive pulmonary disease (COPD) is held responsible for narrowing of small airways and thus for disease progression. Oxidant damage and cell senescence factors are recently involved in airways fibrotic remodeling. Unfortunately, we have no indications on their sequential expression at anatomical sites in which fibrotic remodeling develops in smoking subjects. Using immunohistochemical techniques, we investigated in two strains of mice after cigarette smoke (CS) exposure what happens at various times in airway areas where fibrotic remodeling occurs, and if there also exists correspondence among DNA damage induced by oxidants, cellular senescence, the presence of senescence-secreted factors involved in processes that affect transcription, metabolism as well as apoptosis, and the onset of fibrous remodeling that appears at later times in mice exposed to CS. A clear positivity for fibrogenic cytokines TGF-β, PDGF-B, and CTGF, and for proliferation marker PCNA around airways that will be remodeled is observed in both strains. Increased expression of p16ink4A senescence marker and MyoD is also seen in the same areas. p16ink4A and MyoD can promote cell cycle arrest, terminal differentiation of myofibroblasts, and can oppose their dedifferentiation. Of interest, an early progressive attenuation of SIRT-1 is observed after CS exposure. This intracellular regulatory protein can reduce premature cell senescence. These findings suggest that novel agents, which promote myofibroblast dedifferentiation and/or the apoptosis of senescent cells, may dampen progression of airway changes in smoking COPD subjects.
Keywords: airways changes; animal model; cell senescence; cigarette smoke; histone deacetylase SIRT-1; oxidative DNA damage; proliferation markers.
Conflict of interest statement
Competing InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







References
-
- Mannino DM, Watt G, Hole D, Gillis C, Hart C, McConnachie A, Davey Smith G, Upton M, Hawthorne V, Sin DD, Man SF, Van Eeden S, Mapel DW, Vestbo J. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:627–43. - PubMed
-
- Cigarette smoking and health. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:861–5. - PubMed
-
- Rennard SI, Locantore N, Delafont B, Tal-Singer R, Silverman EK, Vestbo J, Miller BE, Bakke P, Celli B, Calverley PM, Coxson H, Crim C, Edwards LD, Lomas DA, MacNee W, Wouters EF, Yates JC, Coca I, Agustí A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12(3):303–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

