Normative Modeling of Brain Morphometry in Clinical High Risk for Psychosis
- PMID: 37819650
- PMCID: PMC10568447
- DOI: 10.1001/jamapsychiatry.2023.3850
Normative Modeling of Brain Morphometry in Clinical High Risk for Psychosis
Abstract
Importance: The lack of robust neuroanatomical markers of psychosis risk has been traditionally attributed to heterogeneity. A complementary hypothesis is that variation in neuroanatomical measures in individuals at psychosis risk may be nested within the range observed in healthy individuals.
Objective: To quantify deviations from the normative range of neuroanatomical variation in individuals at clinical high risk for psychosis (CHR-P) and evaluate their overlap with healthy variation and their association with positive symptoms, cognition, and conversion to a psychotic disorder.
Design, setting, and participants: This case-control study used clinical-, IQ-, and neuroimaging software (FreeSurfer)-derived regional measures of cortical thickness (CT), cortical surface area (SA), and subcortical volume (SV) from 1340 individuals with CHR-P and 1237 healthy individuals pooled from 29 international sites participating in the Enhancing Neuroimaging Genetics Through Meta-analysis (ENIGMA) Clinical High Risk for Psychosis Working Group. Healthy individuals and individuals with CHR-P were matched on age and sex within each recruitment site. Data were analyzed between September 1, 2021, and November 30, 2022.
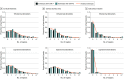
Main outcomes and measures: For each regional morphometric measure, deviation scores were computed as z scores indexing the degree of deviation from their normative means from a healthy reference population. Average deviation scores (ADS) were also calculated for regional CT, SA, and SV measures and globally across all measures. Regression analyses quantified the association of deviation scores with clinical severity and cognition, and 2-proportion z tests identified case-control differences in the proportion of individuals with infranormal (z < -1.96) or supranormal (z > 1.96) scores.
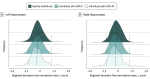
Results: Among 1340 individuals with CHR-P, 709 (52.91%) were male, and the mean (SD) age was 20.75 (4.74) years. Among 1237 healthy individuals, 684 (55.30%) were male, and the mean (SD) age was 22.32 (4.95) years. Individuals with CHR-P and healthy individuals overlapped in the distributions of the observed values, regional z scores, and all ADS values. For any given region, the proportion of individuals with CHR-P who had infranormal or supranormal values was low (up to 153 individuals [<11.42%]) and similar to that of healthy individuals (<115 individuals [<9.30%]). Individuals with CHR-P who converted to a psychotic disorder had a higher percentage of infranormal values in temporal regions compared with those who did not convert (7.01% vs 1.38%) and healthy individuals (5.10% vs 0.89%). In the CHR-P group, only the ADS SA was associated with positive symptoms (β = -0.08; 95% CI, -0.13 to -0.02; P = .02 for false discovery rate) and IQ (β = 0.09; 95% CI, 0.02-0.15; P = .02 for false discovery rate).
Conclusions and relevance: In this case-control study, findings suggest that macroscale neuromorphometric measures may not provide an adequate explanation of psychosis risk.
Conflict of interest statement
Figures


Update of
-
Normative modeling of brain morphometry in Clinical High-Risk for Psychosis.bioRxiv [Preprint]. 2023 Jan 18:2023.01.17.523348. doi: 10.1101/2023.01.17.523348. bioRxiv. 2023. Update in: JAMA Psychiatry. 2024 Jan 1;81(1):77-88. doi: 10.1001/jamapsychiatry.2023.3850. PMID: 36711551 Free PMC article. Updated. Preprint.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
-
- GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi:10.1016/S2215-0366(21)00395-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- MR/L011689/1/MRC_/Medical Research Council/United Kingdom
- R01 MH129636/MH/NIMH NIH HHS/United States
- T32 MH122394/MH/NIMH NIH HHS/United States
- UL1 TR004419/TR/NCATS NIH HHS/United States
- R21 MH117434/MH/NIMH NIH HHS/United States
- R01 MH105246/MH/NIMH NIH HHS/United States
- R01 MH113533/MH/NIMH NIH HHS/United States
- R01 MH115332/MH/NIMH NIH HHS/United States
- T32 MH015144/MH/NIMH NIH HHS/United States
- K23 MH085063/MH/NIMH NIH HHS/United States
- R01 MH105246/MH/NIMH NIH HHS/United States
- R01 MH076989/MH/NIMH NIH HHS/United States
- R01 MH100043/MH/NIMH NIH HHS/United States
- R01 MH113564/MH/NIMH NIH HHS/United States
- R01 MH123163/MH/NIMH NIH HHS/United States
- R01 MH116147/MH/NIMH NIH HHS/United States
- R01 MH115031/MH/NIMH NIH HHS/United States
- K01 MH112774/MH/NIMH NIH HHS/United States
- U54 EB020403/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

