Concordance of a High Lipoprotein(a) Concentration Among Relatives
- PMID: 37819667
- PMCID: PMC10568442
- DOI: 10.1001/jamacardio.2023.3548
Concordance of a High Lipoprotein(a) Concentration Among Relatives
Abstract
Importance: Lipoprotein(a) (Lp[a]) concentrations are a highly heritable and potential causal risk factor for atherosclerotic cardiovascular disease (ASCVD). Recent consensus statements by the European Atherosclerosis Society and American Heart Association recommend screening of relatives of individuals with high Lp(a) concentrations, but the expected yield of this approach has not been quantified in large populations.
Objective: To measure the prevalence of high Lp(a) concentrations among first- and second-degree relatives of individuals with high Lp(a) concentrations compared with unrelated participants.
Design, setting, and participants: In this cross-sectional analysis, pairs of first-degree (n = 19 899) and second-degree (n = 9715) relatives with measured Lp(a) levels from the UK Biobank study and random pairs of unrelated individuals (n = 184 764) were compared. Data for this study were collected from March 2006 to August 2010 and analyzed from December 2021 to August 2023.
Exposure: Serum Lp(a) levels, with a high Lp(a) level defined as at least 125 nmol/L.
Main outcome and measure: Concordance of clinically relevant high Lp(a) levels in first- and second-degree relatives of index participants with high Lp(a) levels.
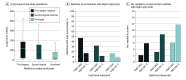
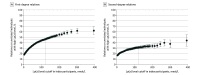
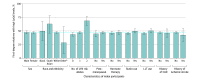
Results: A total of 52 418 participants were included in the analysis (mean [SD] age, 57.3 [8.0] years; 29 825 [56.9%] women). Levels of Lp(a) were correlated among pairs of first-degree (Spearman ρ = 0.45; P < .001) and second-degree (Spearman ρ = 0.22; P < .001) relatives. A total of 1607 of 3420 (47.0% [95% CI, 45.3%-48.7%]) first-degree and 514 of 1614 (31.8% [95% CI, 29.6%-34.2%]) second-degree relatives of index participants with high Lp(a) levels also had elevated concentrations compared with 4974 of 30 258 (16.4% [95% CI, 16.0%-16.9%]) pairs of unrelated individuals. The concordance in high Lp(a) levels was generally consistent among subgroups (eg, those with prior ASCVD, postmenopausal women, and statin users). The odds ratios for relatives to have high Lp(a) levels if their index relative had a high Lp(a) level compared with those whose index relatives did not have high Lp(a) levels were 7.4 (95% CI, 6.8-8.1) for first-degree relatives and 3.0 (95% CI, 2.7-3.4) for second-degree relatives.
Conclusions and relevance: The findings of this cross-sectional study suggest that the yield of cascade screening of first-degree relatives of individuals with high Lp(a) levels is over 40%. These findings support recent recommendations to use this approach to identify additional individuals at ASCVD risk based on Lp(a) concentrations.
Conflict of interest statement
Figures
References
-
- Assessing the Impact of Lipoprotein (a) Lowering With Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients With CVD (Lp[a]HORIZON). ClinicalTrials.gov Identifier: NCT04023552. Updated August 8, 2023. Accessed July 4, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04023552
-
- Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction (OCEAN[a])—Outcomes Trial. ClinicalTrials.gov Identifier: NCT05581303. Updated July 27, 2023. Accessed July 4, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05581303
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

