Digital Health Technology for Real-World Clinical Outcome Measurement Using Patient-Generated Data: Systematic Scoping Review
- PMID: 37819698
- PMCID: PMC10600647
- DOI: 10.2196/46992
Digital Health Technology for Real-World Clinical Outcome Measurement Using Patient-Generated Data: Systematic Scoping Review
Abstract
Background: Digital health technologies (DHTs) play an ever-expanding role in health care management and delivery. Beyond their use as interventions, DHTs also serve as a vehicle for real-world data collection to characterize patients, their care journeys, and their responses to other clinical interventions. There is a need to comprehensively map the evidence-across all conditions and technology types-on DHT measurement of patient outcomes in the real world.
Objective: We aimed to investigate the use of DHTs to measure real-world clinical outcomes using patient-generated data.
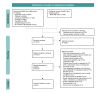
Methods: We conducted this systematic scoping review in accordance with the Joanna Briggs Institute methodology. Detailed eligibility criteria documented in a preregistered protocol informed a search strategy for the following databases: MEDLINE (Ovid), CINAHL, Cochrane (CENTRAL), Embase, PsycINFO, ClinicalTrials.gov, and the EU Clinical Trials Register. We considered studies published between 2000 and 2022 wherein digital health data were collected, passively or actively, from patients with any specified health condition outside of clinical visits. Categories for key concepts, such as DHT type and analytical applications, were established where needed. Following screening and full-text review, data were extracted and analyzed using predefined fields, and findings were reported in accordance with established guidelines.
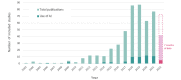
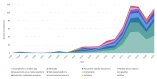
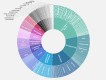
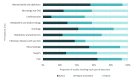
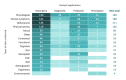
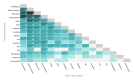
Results: The search strategy identified 11,015 publications, with 7308 records after duplicates and reviews were removed. After screening and full-text review, 510 studies were included for extraction. These studies encompassed 169 different conditions in over 20 therapeutic areas and 44 countries. The DHTs used for mental health and addictions research (111/510, 21.8%) were the most prevalent. The most common type of DHT, mobile apps, was observed in approximately half of the studies (250/510, 49%). Most studies used only 1 DHT (346/510, 67.8%); however, the majority of technologies used were able to collect more than 1 type of data, with the most common being physiological data (189/510, 37.1%), clinical symptoms data (188/510, 36.9%), and behavioral data (171/510, 33.5%). Overall, there has been real growth in the depth and breadth of evidence, number of DHT types, and use of artificial intelligence and advanced analytics over time.
Conclusions: This scoping review offers a comprehensive view of the variety of types of technology, data, collection methods, analytical approaches, and therapeutic applications within this growing body of evidence. To unlock the full potential of DHT for measuring health outcomes and capturing digital biomarkers, there is a need for more rigorous research that goes beyond technology validation to demonstrate whether robust real-world data can be reliably captured from patients in their daily life and whether its capture improves patient outcomes. This study provides a valuable repository of DHT studies to inform subsequent research by health care providers, policy makers, and the life sciences industry.
Trial registration: Open Science Framework 5TMKY; https://osf.io/5tmky/.
Keywords: clinical intervention; digital biomarkers; digital health; digital health management; digital tools; electronic health record; health outcomes; mHealth; mobile health; mobile phone; patient-generated health data; real-world data; real-world evidence; wearables.
©Evelyn Pyper, Sarah McKeown, Jamie Hartmann-Boyce, John Powell. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 11.10.2023.
Conflict of interest statement
Conflicts of Interest: EP is an employee of PicnicHealth, a patient-centric real-world data company. SM works at the Pharmaceutical Research and Manufacturers of America, a trade association representing US pharmaceutical companies in the innovative space. All other authors declare no other conflicts of interest.
Figures
Similar articles
-
Professional-Facing Digital Health Technology for the Care of Patients With Chronic Pain: Scoping Review.J Med Internet Res. 2025 May 14;27:e66457. doi: 10.2196/66457. J Med Internet Res. 2025. PMID: 40367507 Free PMC article.
-
Lessons for Vietnam on the Use of Digital Technologies to Support Patient-Centered Care in Low- and Middle-Income Countries in the Asia-Pacific Region: Scoping Review.J Med Internet Res. 2023 Apr 5;25:e43224. doi: 10.2196/43224. J Med Internet Res. 2023. PMID: 37018013 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Use of Digital Health Technologies for Dementia Care: Bibliometric Analysis and Report.JMIR Ment Health. 2025 Feb 10;12:e64445. doi: 10.2196/64445. JMIR Ment Health. 2025. PMID: 39928936 Free PMC article.
-
Assessing and Enhancing Nutrition and Physical Activity Environments in Early Childhood Education and Care Centers: Scoping Review of eHealth Tools.JMIR Pediatr Parent. 2025 Jan 22;8:e68372. doi: 10.2196/68372. JMIR Pediatr Parent. 2025. PMID: 39841984 Free PMC article. Review.
Cited by
-
Attitudes of healthcare professionals and researchers toward wearable and app derived patient generated health data.NPJ Digit Med. 2025 Mar 30;8(1):186. doi: 10.1038/s41746-025-01568-4. NPJ Digit Med. 2025. PMID: 40159538 Free PMC article.
-
Exploring the Potential of Electronic Patient-Generated Health Data for Evaluating Treatment Response to Intramuscular Steroids in Rheumatoid Arthritis: Case Series.JMIR Form Res. 2024 Oct 28;8:e55715. doi: 10.2196/55715. JMIR Form Res. 2024. PMID: 39467551 Free PMC article.
-
Editorial: Digital health technologies for shared decision making.Front Digit Health. 2025 May 7;7:1574102. doi: 10.3389/fdgth.2025.1574102. eCollection 2025. Front Digit Health. 2025. PMID: 40400541 Free PMC article. No abstract available.
-
Harnessing digital health technologies and real-world evidence to enhance clinical research and patient outcomes.Digit Health. 2025 Jul 23;11:20552076251362097. doi: 10.1177/20552076251362097. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40718403 Free PMC article. Review.
-
Person-centred care, a core concept of family medicine.Eur J Gen Pract. 2024 Dec;30(1):2393860. doi: 10.1080/13814788.2024.2393860. Epub 2024 Aug 27. Eur J Gen Pract. 2024. PMID: 39190294 Free PMC article. No abstract available.
References
-
- Shapiro M, Johnston D, Wald J, Mon D. Patient-generated health data. RTI International. 2012. [2023-01-22]. https://www.rti.org/publication/patient-generated-health-data-white-paper .
-
- Digital health technologies for remote data acquisition in clinical investigations. U.S. Food and Drug Administration. 2021. Dec, [2023-01-03]. https://www.fda.gov/media/155022/download .
-
- Makady A, de Boer A, Hillege H, Klungel O, Goettsch W, (on behalf of GetReal Work Package 1) What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 2017;20(7):858–65. doi: 10.1016/j.jval.2017.03.008. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(17)30171-7 S1098-3015(17)30171-7 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

