A nerve-goblet cell association promotes allergic conjunctivitis through rapid antigen passage
- PMID: 37819721
- PMCID: PMC10721269
- DOI: 10.1172/jci.insight.168596
A nerve-goblet cell association promotes allergic conjunctivitis through rapid antigen passage
Abstract
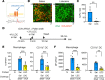
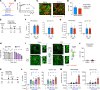
The penetration of allergens through the epithelial layer is the initial step in the development of allergic conjunctivitis. Although pollinosis patients manifest symptoms within minutes after pollen exposure, the mechanisms of the rapid transport of the allergens remain unclear. In the present study, we found that the instillation of pollen shells rapidly induces a large number of goblet cell-associated antigen passages (GAPs) in the conjunctiva. Antigen acquisition by stromal cells, including macrophages and CD11b+ dendritic cells, correlated with surface GAP formation. Furthermore, a substantial amount of antigen was transported to the stroma during the first 10 minutes of pollen exposure, which was sufficient for the full induction of an allergic conjunctivitis mouse model. This inducible, rapid GAP formation and antigen acquisition were suppressed by topical lidocaine or trigeminal nerve ablation, indicating that the sensory nervous system plays an essential role. Interestingly, pollen shell-stimulated GAP formation was not suppressed by topical atropine, suggesting that the conjunctival GAPs and intestinal GAPs are differentially regulated. These results identify pollen shell-induced GAP as a therapeutic target for allergic conjunctivitis.
Keywords: Allergy; Immunology; Ophthalmology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

