Lethal Phenotype-Based Database Screening Identifies Ceramide as a Negative Regulator of Primitive Streak Formation
- PMID: 37819786
- PMCID: PMC10722545
- DOI: 10.1093/stmcls/sxad071
Lethal Phenotype-Based Database Screening Identifies Ceramide as a Negative Regulator of Primitive Streak Formation
Abstract
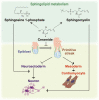
In early embryogenesis, the primitive streak (PrS) generates the mesendoderm and is essential for organogenesis. However, because the PrS is a minute and transient tissue, elucidating the mechanism of its formation has been challenging. We performed comprehensive screening of 2 knockout mouse databases based on the fact that failure of PrS formation is lethal. We identified 812 genes involved in various cellular functions and responses that might be linked to PrS formation, with the category of greatest abundance being "Metabolism." In this study, we focused on genes of sphingolipid metabolism and investigated their roles in PrS formation using an in vitro mouse ES cell differentiation system. We show here that elevated intracellular ceramide negatively regulates gene expression essential for PrS formation and instead induces neurogenesis. In addition, sphingosine-1-phosphate (a ceramide derivative) positively regulates neural maturation. Our results indicate that ceramide regulates both PrS formation and the induction of neural differentiation.
Keywords: cardiac differentiation; ceramide; neural differentiation; primitive streak; sphingosine-1-phosphate.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures






References
-
- Desbaillets I, Ziegler U, Groscurth P, Gassmann M.. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85(6):645-651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

