Phase II Trial of Nivolumab in Metastatic Rare Cancer with dMMR or MSI-H and Relation with Immune Phenotypic Analysis (the ROCK Trial)
- PMID: 37819940
- PMCID: PMC10722134
- DOI: 10.1158/1078-0432.CCR-23-1807
Phase II Trial of Nivolumab in Metastatic Rare Cancer with dMMR or MSI-H and Relation with Immune Phenotypic Analysis (the ROCK Trial)
Abstract
Purpose: Mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) are positive predictive markers for immune checkpoint inhibitors. However, data on the activity of nivolumab in advanced dMMR/MSI-H rare cancers and more accurate biomarkers are worth exploring.
Patients and methods: We conducted a multicenter phase II, open-label, single-arm clinical trial to explore the effectiveness and safety of nivolumab monotherapy in patients with advanced rare cancers with dMMR/MSI-H, in parallel with immune phenotype analysis, to explore new biomarkers. A Bayesian adaptive design was applied. Characterization of peripheral blood mononuclear cells (PBMC) was characterized by multicolor flow cytometric analysis and CyTOF using samples collected before and after the intervention. The dMMR was identified by the complete loss of MLH1/MSH2/MSH6/PMS2.
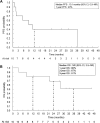
Results: From May 2018 to March 2021, 242 patients were screened, and 11 patients were enrolled, of whom 10 were included in the full analysis. Median follow-up was 24.7 months (interquartile range, 12.4-31.5). Objective response rate was 60% [95% confidence interval (CI), 26.2-87.8] by central assessment and 70% (95% CI, 34.8-93.3) by local investigators. Median progression-free survival was 10.1 months (95% CI, 0.9-11.1). No treatment-related adverse events of grade 3 or higher were observed. Patients with a tumor mutation burden of ≥10/Mb showed a 100% response rate (95% CI, 47.8-100). Responders had increased T-bet+ PD-1+ CD4+ T cells in PBMC compared with nonresponders (P < 0.05).
Conclusions: The trial met its primary endpoint with nivolumab, demonstrating clinical benefit in advanced dMMR/MSI-H rare solid cancers. Besides, the proportion of T-bet+ PD-1+ CD4+ T-cells may serve as a novel predictive biomarker.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 2015;1:1319–23. - PubMed
-
- Huang RSP, Haberberger J, Severson E, Duncan DL, Hemmerich A, Edgerly C, et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden, and microsatellite instability in 48,782 cases. Mod Pathol 2021;34:252–63. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

