FAK Drives Resistance to Therapy in HPV-Negative Head and Neck Cancer in a p53-Dependent Manner
- PMID: 37819945
- PMCID: PMC10767302
- DOI: 10.1158/1078-0432.CCR-23-0964
FAK Drives Resistance to Therapy in HPV-Negative Head and Neck Cancer in a p53-Dependent Manner
Abstract
Purpose: Radiation and platinum-based chemotherapy form the backbone of therapy in human papillomavirus (HPV)-negative head and neck squamous cell carcinoma (HNSCC). We have correlated focal adhesion kinase (FAK/PTK2) expression with radioresistance and worse outcomes in these patients. However, the importance of FAK in driving radioresistance and its effects on chemoresistance in these patients remains unclear.
Experimental design: We performed an in vivo shRNA screen using targetable libraries to identify novel therapeutic sensitizers for radiation and chemotherapy.
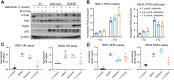
Results: We identified FAK as an excellent target for both radio- and chemosensitization. Because TP53 is mutated in over 80% of HPV-negative HNSCC, we hypothesized that mutant TP53 may facilitate FAK-mediated therapy resistance. FAK inhibitor increased sensitivity to radiation, increased DNA damage, and repressed homologous recombination and nonhomologous end joining repair in mutant, but not wild-type, TP53 HPV-negative HNSCC cell lines. The mutant TP53 cisplatin-resistant cell line had increased FAK phosphorylation compared with wild-type, and FAK inhibition partially reversed cisplatin resistance. To validate these findings, we utilized an HNSCC cohort to show that FAK copy number and gene expression were associated with worse disease-free survival in mutant TP53, but not wild-type TP53, HPV-negative HNSCC tumors.
Conclusions: FAK may represent a targetable therapeutic sensitizer linked to a known genomic marker of resistance.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

