Shotgun Kinetic Target-Guided Synthesis Approach Enables the Discovery of Small-Molecule Inhibitors against Pathogenic Free-Living Amoeba Glucokinases
- PMID: 37820055
- PMCID: PMC10644346
- DOI: 10.1021/acsinfecdis.3c00284
Shotgun Kinetic Target-Guided Synthesis Approach Enables the Discovery of Small-Molecule Inhibitors against Pathogenic Free-Living Amoeba Glucokinases
Abstract
Pathogenic free-living amoebae (pFLA) can cause life-threatening central nervous system (CNS) infections and warrant the investigation of new chemical agents to combat the rise of infection from these pathogens. Naegleria fowleri glucokinase (NfGlck), a key metabolic enzyme involved in generating glucose-6-phosphate, was previously identified as a potential target due to its limited sequence similarity with human Glck (HsGlck). Herein, we used our previously demonstrated multifragment kinetic target-guided synthesis (KTGS) screening strategy to identify inhibitors against pFLA glucokinases. Unlike the majority of previous KTGS reports, our current study implements a "shotgun" approach, where fragments were not biased by predetermined binding potentials. The study resulted in the identification of 12 inhibitors against 3 pFLA glucokinase enzymes─NfGlck, Balamuthia mandrillaris Glck (BmGlck), and Acanthamoeba castellanii Glck (AcGlck). This work demonstrates the utility of KTGS to identify small-molecule binders for biological targets where resolved X-ray crystal structures are not readily accessible.
Keywords: glucokinases; kinetic target-guided synthesis; multifragment screening; small molecule inhibitors; sulfo-click reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
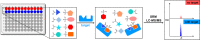
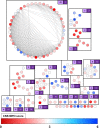

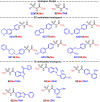
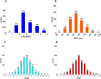

References
-
- Visvesvara G. S. Free-Living Amebae as Opportunistic Agents of Human Disease. J. Neuroparasitology 2010, 1, N100802.10.4303/jnp/N100802. - DOI
-
- Capewell L. G.; Harris A. M.; Yoder J. S.; Cope J. R.; Eddy B. A.; Roy S. L.; Visvesvara G. S.; Fox L. M.; Beach M. J. Diagnosis, Clinical Course, and Treatment of Primary Amoebic Meningoencephalitis in the United States, 1937–2013. J. Pediatr. Infect. Dis. Soc. 2015, 4 (4), e68–e75. 10.1093/jpids/piu103. - DOI - PubMed
-
- Cope J. R.; Conrad D. A.; Cohen N.; Cotilla M.; DaSilva A.; Jackson J.; Visvesvara G. S. Use of the Novel Therapeutic Agent Miltefosine for the Treatment of Primary Amebic Meningoencephalitis: Report of 1 Fatal and 1 Surviving Case. Clin. Infect. Dis. 2016, 62 (6), 774–776. 10.1093/cid/civ1021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

