Dysregulated meta-organismal metabolism of aromatic amino acids in alcohol-associated liver disease
- PMID: 37820283
- PMCID: PMC10578770
- DOI: 10.1097/HC9.0000000000000284
Dysregulated meta-organismal metabolism of aromatic amino acids in alcohol-associated liver disease
Abstract
Background: Chronic alcohol consumption impairs gut barrier function and perturbs the gut microbiome. Although shifts in bacterial communities in patients with alcohol-associated liver disease (ALD) have been characterized, less is known about the interactions between host metabolism and circulating microbe-derived metabolites during the progression of ALD.
Methods: A large panel of gut microbiome-derived metabolites of aromatic amino acids was quantified by stable isotope dilution liquid chromatography with online tandem mass spectrometry in plasma from healthy controls (n = 29), heavy drinkers (n = 10), patients with moderate (n = 16) or severe alcohol-associated hepatitis (n = 40), and alcohol-associated cirrhosis (n = 10).
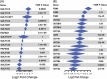
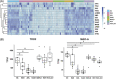
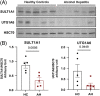
Results: The tryptophan metabolites, serotonin and indole-3-propionic acid, and tyrosine metabolites, p-cresol sulfate, and p-cresol glucuronide, were decreased in patients with ALD. Patients with severe alcohol-associated hepatitis and alcohol-associated cirrhosis had the largest decrease in concentrations of tryptophan and tyrosine-derived metabolites compared to healthy control. Western blot analysis and interrogation of bulk RNA sequencing data from patients with various liver pathologies revealed perturbations in hepatic expression of phase II metabolism enzymes involved in sulfonation and glucuronidation in patients with severe forms of ALD.
Conclusions: We identified several metabolites decreased in ALD and disruptions of hepatic phase II metabolism. These results indicate that patients with more advanced stages of ALD, including severe alcohol-associated hepatitis and alcohol-associated cirrhosis, had complex perturbations in metabolite concentrations that likely reflect both changes in the composition of the gut microbiome community and the ability of the host to enzymatically modify the gut-derived metabolites.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Mack Mitchell is employed and owns stock in Amygdala Neuroscience. He advises HepaTx and Prodigy Biotech and owns stock in AbbVie. Gyongyi Szabo consults for CYTA Therapeutics, Durect, Merck, Pandion, Pfizer, Terra Firma, and Glympse Bio. Stanley Hazen consults, received grants, and holds intellectual property rights with Proctor & Gamble. He consults and received grants from Zehna Therapeutics. He received grants from Roche and holds intellectual property rights with Cleveland HeartLab. The remaining authors have no conflicts to report.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK120679/DK/NIDDK NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- R01 HL144651/HL/NHLBI NIH HHS/United States
- P01 HL147823/HL/NHLBI NIH HHS/United States
- R01 HL103866/HL/NHLBI NIH HHS/United States
- R01 DK130227/DK/NIDDK NIH HHS/United States
- U01 AA021890/AA/NIAAA NIH HHS/United States
- U01 AA026980/AA/NIAAA NIH HHS/United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- R56 HL141744/HL/NHLBI NIH HHS/United States
- U01 AA021893/AA/NIAAA NIH HHS/United States
- U01 AA021901/AA/NIAAA NIH HHS/United States
- P20 GM113226/GM/NIGMS NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- P50 AA024337/AA/NIAAA NIH HHS/United States
- R21 AA026398/AA/NIAAA NIH HHS/United States
- U01 AA026975/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources

