Adult-born granule cells facilitate remapping of spatial and non-spatial representations in the dentate gyrus
- PMID: 37820723
- PMCID: PMC10841867
- DOI: 10.1016/j.neuron.2023.09.016
Adult-born granule cells facilitate remapping of spatial and non-spatial representations in the dentate gyrus
Abstract
Adult-born granule cells (abGCs) have been implicated in memory discrimination through a neural computation known as pattern separation. Here, using in vivo Ca2+ imaging, we examined how chronic ablation or acute chemogenetic silencing of abGCs affects the activity of mature granule cells (mGCs). In both cases, we observed altered remapping of mGCs. Rather than broadly modulating the activity of all mGCs, abGCs promote the remapping of place cells' firing fields while increasing rate remapping of mGCs that represent sensory cues. In turn, these remapping deficits are associated with behavioral impairments in animals' ability to correctly identify new goal locations. Thus, abGCs facilitate pattern separation through the formation of non-overlapping representations for identical sensory cues encountered in different locations. In the absence of abGCs, the dentate gyrus shifts to a state that is dominated by cue information, a situation that is consistent with the overgeneralization often observed in anxiety or age-related disorders.
Keywords: adult neurogenesis; dentate gyrus; in vivo two-photon Ca(2+) imaging; pattern separation.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
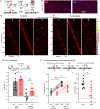
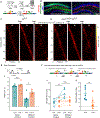

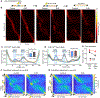
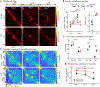

References
Publication types
MeSH terms
Grants and funding
- R01 MH068542/MH/NIMH NIH HHS/United States
- CDP 13-003/HX/HSRD VA/United States
- R01 MH083862/MH/NIMH NIH HHS/United States
- RF1 AG080818/AG/NIA NIH HHS/United States
- K01 AG054765/AG/NIA NIH HHS/United States
- R01 MH100631/MH/NIMH NIH HHS/United States
- R01 NS094668/NS/NINDS NIH HHS/United States
- K99 MH122226/MH/NIMH NIH HHS/United States
- F31 MH012226/MH/NIMH NIH HHS/United States
- R00 MH122226/MH/NIMH NIH HHS/United States
- R37 MH068542/MH/NIMH NIH HHS/United States
- U19 NS104590/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

