A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus
- PMID: 37821426
- PMCID: PMC10567910
- DOI: 10.1038/s41467-023-41877-4
A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus
Abstract
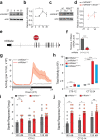
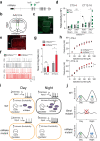
Circadian clocks generate rhythms of arousal, but the underlying molecular and cellular mechanisms remain unclear. In Drosophila, the clock output molecule WIDE AWAKE (WAKE) labels rhythmic neural networks and cyclically regulates sleep and arousal. Here, we show, in a male mouse model, that mWAKE/ANKFN1 labels a subpopulation of dorsomedial hypothalamus (DMH) neurons involved in rhythmic arousal and acts in the DMH to reduce arousal at night. In vivo Ca2+ imaging reveals elevated DMHmWAKE activity during wakefulness and rapid eye movement (REM) sleep, while patch-clamp recordings show that DMHmWAKE neurons fire more frequently at night. Chemogenetic manipulations demonstrate that DMHmWAKE neurons are necessary and sufficient for arousal. Single-cell profiling coupled with optogenetic activation experiments suggest that GABAergic DMHmWAKE neurons promote arousal. Surprisingly, our data suggest that mWAKE acts as a clock-dependent brake on arousal during the night, when mice are normally active. mWAKE levels peak at night under clock control, and loss of mWAKE leads to hyperarousal and greater DMHmWAKE neuronal excitability specifically at night. These results suggest that the clock does not solely promote arousal during an animal's active period, but instead uses opposing processes to produce appropriate levels of arousal in a time-dependent manner.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

