The role of intrathecal free light chains kappa for the detection of autoimmune encephalitis in subacute onset neuropsychiatric syndromes
- PMID: 37821561
- PMCID: PMC10567819
- DOI: 10.1038/s41598-023-44427-6
The role of intrathecal free light chains kappa for the detection of autoimmune encephalitis in subacute onset neuropsychiatric syndromes
Abstract
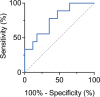
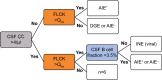
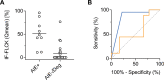
Intrathecal synthesis of free light chains kappa (FLCK) is increasingly recognized as a marker of inflammatory CNS pathologies. Here, we tested the performance of FLCK in differentiating autoimmune encephalitis (AIE) from non-inflammatory etiologies in subacute onset neuropsychiatric syndromes. Patients undergoing diagnostic work-up for suspected autoimmune encephalitis at our department between 2015 and 2020 were retrospectively assessed for definitive diagnosis, available CSF and blood samples, as well as complete clinical records. Intrathecal FLCK was measured along with established CSF markers of CNS inflammation. The study cohort consisted of 19 patients with antibody-mediated AIE (AIE+), 18 patients with suspected AIE but without detectable autoantibodies (AIE-), 10 patients with infectious (viral) encephalitis (INE), and 15 patients with degenerative encephalopathies (DGE). 25 age- and sex-matched patients with non-inflammatory neurological diseases (NIND) were used as a control group. All AIE+ patients exhibited intrathecal synthesis of FLCK compared to only 39% of AIE- patients and 81% of patients in the INE group. No intrathecal synthesis of FLCK was found in DGE and NIND patients. While intrathecal FLCK was equally specific for an inflammatory etiology as oligoclonal bands (OCB) in the cerebrospinal fluid (CSF), the sensitivity of intrathecal FLCK for any inflammatory intrathecal process was higher than that of OCB (83% vs. 38%). Intrathecal FLCK synthesis was found to discriminate AIE+ from non-inflammatory encephalopathies and AIE- when the CSF cell count was normal [receiver operating characteristic (ROC) analysis area under the curve (AUC): 0.867, p = 0.002], while it failed to differentiate between AIE+ and INE in the presence of CSF pleocytosis (AUC: 0.561, p = 0.607). In conclusion, in the absence of CSF pleocytosis, intrathecal FLCK discriminated AIE+ from competing diagnoses in our cohort of subacute onset neuropsychiatric syndromes. In addition to established markers of CSF inflammation, intrathecal FLCK might support clinical decision-making and contribute to selecting patients for (repeated) antibody testing.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

