Apoptotic stress causes mtDNA release during senescence and drives the SASP
- PMID: 37821702
- PMCID: PMC10584674
- DOI: 10.1038/s41586-023-06621-4
Apoptotic stress causes mtDNA release during senescence and drives the SASP
Erratum in
-
Author Correction: Apoptotic stress causes mtDNA release during senescence and drives the SASP.Nature. 2024 Jan;625(7995):E15. doi: 10.1038/s41586-023-07002-7. Nature. 2024. PMID: 38168624 Free PMC article. No abstract available.
Abstract
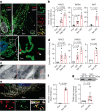
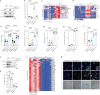
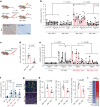
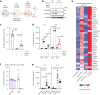
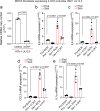
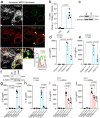
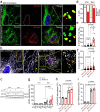
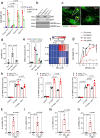
Senescent cells drive age-related tissue dysfunction partially through the induction of a chronic senescence-associated secretory phenotype (SASP)1. Mitochondria are major regulators of the SASP; however, the underlying mechanisms have not been elucidated2. Mitochondria are often essential for apoptosis, a cell fate distinct from cellular senescence. During apoptosis, widespread mitochondrial outer membrane permeabilization (MOMP) commits a cell to die3. Here we find that MOMP occurring in a subset of mitochondria is a feature of cellular senescence. This process, called minority MOMP (miMOMP), requires BAX and BAK macropores enabling the release of mitochondrial DNA (mtDNA) into the cytosol. Cytosolic mtDNA in turn activates the cGAS-STING pathway, a major regulator of the SASP. We find that inhibition of MOMP in vivo decreases inflammatory markers and improves healthspan in aged mice. Our results reveal that apoptosis and senescence are regulated by similar mitochondria-dependent mechanisms and that sublethal mitochondrial apoptotic stress is a major driver of the SASP. We provide proof-of-concept that inhibition of miMOMP-induced inflammation may be a therapeutic route to improve healthspan.
© 2023. The Author(s).
Conflict of interest statement
V.I.K. is a scientific advisor for Longaevus Technologies. S.W.G.T. consults for Exo Therapeutics. The other authors declare no competing interests.
Figures


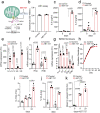
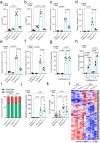
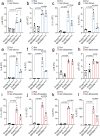

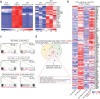
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK128552/DK/NIDDK NIH HHS/United States
- MR/R023026/1/MRC_/Medical Research Council/United Kingdom
- 23390/CRUK_/Cancer Research UK/United Kingdom
- R01 AG076515/AG/NIA NIH HHS/United States
- P30 AG068635/AG/NIA NIH HHS/United States
- P01 AG073084/AG/NIA NIH HHS/United States
- U54 AG079754/AG/NIA NIH HHS/United States
- R01 AG071861/AG/NIA NIH HHS/United States
- F31 AG062099/AG/NIA NIH HHS/United States
- R01 AR069876/AR/NIAMS NIH HHS/United States
- RF1 AG070391/AG/NIA NIH HHS/United States
- UG3 CA268103/CA/NCI NIH HHS/United States
- R33 AG061456/AG/NIA NIH HHS/United States
- DRCPFA-NOV22/100001/CRUK_/Cancer Research UK/United Kingdom
- DRCRPG-NOV22/100007/CRUK_/Cancer Research UK/United Kingdom
- UH3 CA268103/CA/NCI NIH HHS/United States
- P01 AG062413/AG/NIA NIH HHS/United States
- R01 CA225996/CA/NCI NIH HHS/United States
- R01 AG082708/AG/NIA NIH HHS/United States
- R01 AG064165/AG/NIA NIH HHS/United States
- R01 AG068182/AG/NIA NIH HHS/United States
- R01 AG068048/AG/NIA NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
- R01 AG061875/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

