Impact of Risk Minimisation Measures on Valproate Use among Women of Reproductive Age in Latvia Between 2013 and 2020: A 7-Year Nationwide Prescription Database Study
- PMID: 37821776
- PMCID: PMC10730785
- DOI: 10.1007/s40801-023-00394-y
Impact of Risk Minimisation Measures on Valproate Use among Women of Reproductive Age in Latvia Between 2013 and 2020: A 7-Year Nationwide Prescription Database Study
Abstract
Background: A relevant safety concern for the use of valproate (VPA) in women of reproductive age is its teratogenicity. In 2014 European Medicines Agency (EMA) introduced risk minimisation measures (RMMs) to reduce the VPA use by women of reproductive age, where the impact on VPA use was not as large as expected. In 2018, the EMA introduced additional RMMs, and it is essential to assess impact of these interventions.
Objective: The objective of this study was to evaluate the impact of the EMA-published RMMs in 2014 and 2018 on the prevalence of VPA use and to describe trends in the prevalence rate and incidence proportion of VPA use in epilepsy, bipolar disorder and off-label indications in Latvia.
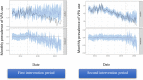
Methods: This was a nationwide population-based study using a primary care prescription database. The study included women in age groups < 15, 15-49 and > 49 years and men in age group 15-49 years who have received VPA. This study assessed the prevalence rate and the incidence proportion of VPA use. The impact of RMMs on the two study intervention periods [fourth quarter (Q4) 2014 and Q4 2018] in men and women was evaluated using causal impact analysis.
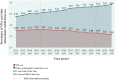
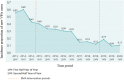
Results: In the study cohort, VPA use in women in the age group 15-49 years decreased after the first and second intervention periods, where after the first intervention period the relative reduction in prevalence of VPA consumption was -7.7 [95% confidence interval (CI) -10%, -5.1%] and after both study periods -6.4% (95% CI -11%, -1.5%). In girls < 15 years of age, valproate use decreased after both intervention periods, while in women > 49 years old VPA use increased. In men aged 15-49 years, an increase after the first period and a non-significant decrease after both intervention periods was observed. The prevalence of valproate use in girls < 15 years and women 15-49 years of age with bipolar disorder, epilepsy and off-label indications decreased per 1000 people during the study period. The incidence proportion of VPA use in women aged 15-49 years decreased each year since the beginning of the study period.
Conclusions: A statistically significant decrease in the prevalence of VPA use was identified among girls < 15 years and women 15-49 years of age. In Latvia, an overall good reaction to the EMA RMMs was observed. The effects go beyond the target population and affect the use of VPA in young girls as well.
© 2023. The Author(s).
Conflict of interest statement
The authors have declared that no competing interests exists.
Figures

References
-
- European Medicines Agency. Assessment report. Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data. Substances related to valproate. Procedure number: EMEA/H/A-31/1387. 2014.
-
- European Medicines Agency. New measures to avoid valproate exposure in pregnancy endorsed. Member State representatives agree new restrictions and pregnancy prevention programme. . EMA/375438/2018 2018. https://www.ema.europa.eu/en/documents/referral/valproate-article-31-ref... (accessed Sep 6, 2021).
LinkOut - more resources
Full Text Sources
Research Materials

