OCOSO2: study protocol for a single-blinded, multicentre, randomised controlled trial assessing a central venous oxygen saturation-based goal-directed therapy to reduce postoperative complications in high-risk patients after elective major surgery
- PMID: 37821968
- PMCID: PMC10568773
- DOI: 10.1186/s13063-023-07689-z
OCOSO2: study protocol for a single-blinded, multicentre, randomised controlled trial assessing a central venous oxygen saturation-based goal-directed therapy to reduce postoperative complications in high-risk patients after elective major surgery
Abstract
Background: Fluid loading-based goal-directed therapy is a cornerstone of anaesthesia management in major surgery. Its widespread application has contributed to a significant improvement in perioperative morbidity and mortality. In theory, only hypovolemic patients should receive fluid therapy. However, to achieve such a diagnosis, a surrogate marker of cardiac output adequacy must be used. Current methods of fluid loading-based goal-directed therapy do not assess cardiac output adequacy. Nowadays, new devices make it possible to continuously monitor central venous oxygen saturation (ScvO2) and therefore, to assess the adequacy of perioperative cardiac output during surgery. In major surgery, ScvO2-based goal-directed therapy can be used to enhance fluid therapy and improve patient outcomes.
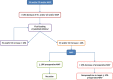
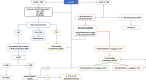
Methods: We designed a prospective, randomised, single-blinded, multicentre controlled superiority study with a 1:1 allocation ratio. Patients to be included will be high-risk major surgery patients (> 50 years old, ASA score > 2, major intra-abdominal or intra-thoracic surgery > 90 min). Patients in the control group will undergo standard fluid loading-based goal-directed therapy, as recommended by the guidelines. Patients in the intervention group will have ScvO2-based goal-directed therapy and receive fluid loading only if fluid responsiveness and cardiac output inadequacy are present. The primary outcome will be the Comprehensive Complication Index on day five postoperatively.
Discussion: This study is the first to address the issue of cardiac output adequacy in goal-directed therapy. Our hypothesis is that cardiac output optimisation during major surgery achieved by continuous monitoring of the ScvO2 to guide fluid therapy will result in a reduction of postoperative complications as compared with current goal-directed fluid therapy practices.
Trial registration: ClinicalTrials.gov. NCT03828565. Registered on February 4, 2019.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
Professor Leone reports personal fees from Aspen, AMOMED, LFB, Ambu and Gilead outside of the submitted work.
Professor Zieleskiewicz reports personal fees from General Electric Healthcare outside of the submitted work.
Dr Pastene reports personal fees from Edwards Lifesciences outside of the submitted work.
Dr Bernat, Dr Baumstark, Dr Bezulier, Dr Gricourt, Dr De Guibert, Dr Charvet and Dr Colin have nothing to disclose.
Figures
References
-
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth. 2002;88(1):65–71. doi: 10.1093/bja/88.1.65. - DOI - PubMed
-
- Mukai A, Suehiro K, Watanabe R, Juri T, Hayashi Y, Tanaka K, et al. Impact of intraoperative goal-directed fluid therapy on major morbidity and mortality after transthoracic oesophagectomy: a multicentre, randomised controlled trial. Br J Anaesth. 2020;125(6):953–961. doi: 10.1016/j.bja.2020.08.060. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

