Allogeneic tumor cell-derived extracellular vesicles stimulate CD8 T cell response in colorectal cancer
- PMID: 37822487
- PMCID: PMC10562189
- DOI: 10.1016/j.omto.2023.100727
Allogeneic tumor cell-derived extracellular vesicles stimulate CD8 T cell response in colorectal cancer
Abstract
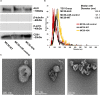
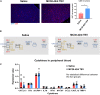
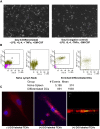
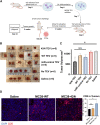
Most colorectal cancer (CRC) patients present with a microsatellite-stable phenotype, rendering them resistant to immune checkpoint inhibitors (ICIs). Among the contributors to ICI resistance, tumor-derived extracellular vesicles (TEVs) have emerged as critical players. Previously we demonstrated that autologous transfer of TEVs without miR-424 can induce tumor antigen-specific immune responses in CRC models. Therefore, we postulated that allogeneic TEVs, modified to lack miR-424 and derived from an MC38 cells, could induce CD8+ T cell responses while restraining CT26 cell-based tumor. Here, we show that prophylactic administration of MC38 TEVs, without miR-424, showed a significant augmentation in CD8+ T-cells within CT26 tumors. This allogenic TEV effect was evident in CT26 tumors but not B16-F10 melanoma. Furthermore, we demonstrated the capacity of dendritic cells (DCs) to internalize TEVs, a possible mechanism to elicit immune response. Our investigation of autologously administered DCs, which had been exposed to modified TEVs, underscores their potential to dampen tumor growth while elevating CD8+ T cell levels vis-a-vis MC38 wild-type TEVs exposed to DCs. Notably, the modified TEVs were well tolerated and did not increase peripheral blood cytokine levels. Our findings underscore the potential of modified allogeneic TEVs without immune-suppressive factors to elicit robust T cell responses and limit tumor growth.
Keywords: T cells; allogeneic; colorectal cancer; dendritic cells; immune checkpoint inhibitors; tumor extracellular vesicles.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Allogeneic Tumor Cell-Derived Extracellular Vesicles Stimulate CD8 T Cell Response in Colorectal Cancer.bioRxiv [Preprint]. 2023 Apr 18:2023.04.17.537250. doi: 10.1101/2023.04.17.537250. bioRxiv. 2023. Update in: Mol Ther Oncolytics. 2023 Sep 16;31:100727. doi: 10.1016/j.omto.2023.100727. PMID: 37131597 Free PMC article. Updated. Preprint.
References
-
- Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.W., Carcereny Costa E., Park K., Alexandru A., Lupinacci L., de la Mora Jimenez E., et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

