A modular phage vector platform for targeted photodynamic therapy of Gram-negative bacterial pathogens
- PMID: 37822492
- PMCID: PMC10563061
- DOI: 10.1016/j.isci.2023.108032
A modular phage vector platform for targeted photodynamic therapy of Gram-negative bacterial pathogens
Abstract
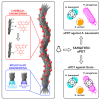
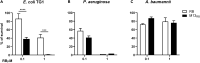
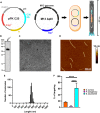
Growing antibiotic resistance has encouraged the revival of phage-inspired antimicrobial approaches. On the other hand, photodynamic therapy (PDT) is considered a very promising research domain for the protection against infectious diseases. Yet, very few efforts have been made to combine the advantages of both approaches in a modular, retargetable platform. Here, we foster the M13 bacteriophage as a multifunctional scaffold, enabling the selective photodynamic killing of bacteria. We took advantage of the well-defined molecular biology of M13 to functionalize its capsid with hundreds of photo-activable Rose Bengal sensitizers and contemporarily target this light-triggerable nanobot to specific bacterial species by phage display of peptide targeting moieties fused to the minor coat protein pIII of the phage. Upon light irradiation of the specimen, the targeted killing of diverse Gram(-) pathogens occurred at subnanomolar concentrations of the phage vector. Our findings contribute to the development of antimicrobials based on targeted and triggerable phage-based nanobiotherapeutics.
Keywords: Bacteriology; Biotechnology; Microbiology; Virology.
© 2023 The Author(s).
Conflict of interest statement
There are no conflicts to declare.
Figures

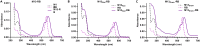


References
-
- Uyttebroek S., Chen B., Onsea J., Ruythooren F., Debaveye Y., Devolder D., Spriet I., Depypere M., Wagemans J., Lavigne R., et al. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect. Dis. 2022;22:e208–e220. doi: 10.1016/S1473-3099(21)00612-5. - DOI - PubMed
-
- Schooley R.T., Biswas B., Gill J.J., Hernandez-Morales A., Lancaster J., Lessor L., Barr J.J., Reed S.L., Rohwer F., Benler S., et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00954-17. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

