The Therapeutic Effects of MUC1-C shRNA@Fe3O4 Magnetic Nanoparticles in Alternating Magnetic Fields on Triple-Negative Breast Cancer
- PMID: 37822991
- PMCID: PMC10563812
- DOI: 10.2147/IJN.S426849
The Therapeutic Effects of MUC1-C shRNA@Fe3O4 Magnetic Nanoparticles in Alternating Magnetic Fields on Triple-Negative Breast Cancer
Abstract
Purpose: Improving the treatment of triple-negative breast cancer (TNBC) is a serious challenge today. The primary objective of this study was to construct MUC1-C shRNA@ Fe3O4 magnetic nanoparticles (MNPs) and investigate their potential therapeutic benefits in alternating magnetic fields (AMF) on TNBC.
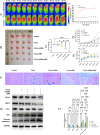
Methods: Firstly, we verified the high expression of MUC1 in TNBC and synthesized specific MUC1-C shRNA plasmids (MUC1-C shRNA). Then, we prepared and characterized MUC1-C shRNA@Fe3O4 MNPs and confirmed their MUC1-C gene silencing effect and magneto-thermal conversion ability in AMF. Moreover, the inhibitory effects on TNBC in vitro and in vivo were observed as well as biosafety. Finally, the protein levels of BCL-2-associated X protein (Bax), cleaved-caspase3, glutathione peroxidase inhibitor 4 (GPX4), nuclear factor erythroid 2-related factor 2 (NRF2), and ferritin heavy chain 1 (FTH1) in TNBC cells and tissues were examined, and it was speculated that apoptosis and ferroptosis were involved in the synergistic treatment.
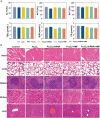
Results: MUC1-C shRNA@ Fe3O4 MNPs have a size of ~75 nm, with an encapsulation rate of (29.78±0.63) %, showing excellent gene therapy and magnetic hyperthermia functions. Under a constant AMF (3Kw) and a set concentration (200µg mL-1), the nanoparticles could be rapidly warmed up within 20 minutes and stabilized at about 43 °C. It could be uptaken by TNBC cells through endocytosis and significantly inhibit their proliferation and migration, with a growth inhibition rate of 79.22% for TNBC tumors. After treatment, GPX4, NRF2, and FTH1 expression levels in TNBC cells and tumor tissues were suppressed, while Bax and cleaved-caspase3 were increased. As key therapeutic measures, gene therapy, and magnetic hyperthermia have shown a synergistic effect in this treatment strategy, with a combined index (q index) of 1.23.
Conclusion: In conclusion, we developed MUC1-C shRNA@Fe3O4 MNPs with magnetic hyperthermia and gene therapy functions, which have shown satisfactory therapeutic effects on TNBC without significant side effects. This study provides a potential option for the precision treatment of TNBC.
Keywords: MUC1; ferroptosis; hyperthermia; nanoparticle; triple-negative breast cancer.
© 2023 Li et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
