Mango ginger (Curcuma amada Roxb.) may alleviate the effect of high-fat diet/streptozotocin-induced diabetes by activation of the GSK-3β/Fyn/Nrf2 pathway
- PMID: 37823118
- PMCID: PMC10563713
- DOI: 10.1002/fsn3.3539
Mango ginger (Curcuma amada Roxb.) may alleviate the effect of high-fat diet/streptozotocin-induced diabetes by activation of the GSK-3β/Fyn/Nrf2 pathway
Abstract
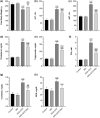
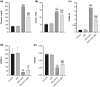
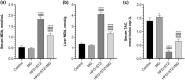
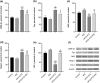
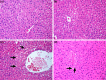
Mango ginger (MG) exhibits antioxidant, anti-inflammatory, and antihyperglycemic effects; however, the exact mechanism of action of MG extract in relation to its antidiabetic properties remains unclear. To investigate the potential antidiabetic effect of MG extract, we used a high-fat diet (HFD)/low-dose streptozotocin (STZ)-induced type 2 diabetic rat model. A total of 28 male Wistar rats were randomly divided into four groups: (i) Control, (ii) MG (50 mg/kg/day of MG extract), (iii) HFD + STZ (40 mg/kg i.p.), and (iv) HFD + STZ + MG. Following a 12-week administration of MG extract, significant reductions were observed in serum glucose, insulin, free fatty acid, cholesterol, and triglyceride levels in diabetic rats (p < .0001 for all). MG extract supplementation led to an increase in the total antioxidant capacity of the serum and a decrease in malondialdehyde (MDA) levels in both the serum and liver (p < .0001). Furthermore, hepatocellular fat accumulation was partially attenuated in the HFD + STZ + MG group. Notably, MG extract inhibited glycogen synthase kinase-3β (GSK-3β) in the liver (p < .01) and downregulated Fyn expression, resulting in elevated nuclear factor erythroid 2-related factor 2 (Nrf2) activity in the HFD + STZ + MG group compared to the HFD + STZ group (p < .05). The increased activity of Nrf2 in the HFD + STZ + MG group likely promoted the upregulation of heme oxygenase 1 (HO-1) in the liver (p < .0001). In conclusion, MG extract may exert antidiabetic effects by augmenting the antioxidant defense system through the regulation of GSK-3β/Fyn/Nrf2 in a rat model of type 2 diabetes.
Keywords: Nrf2; antioxidant capacity; diabetes; insulin resistance; mango ginger.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they do not have any conflict of interest.
Figures
Similar articles
-
Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3β activity and activating the Nrf2/HO-1 pathway.Phytomedicine. 2022 Jan;95:153856. doi: 10.1016/j.phymed.2021.153856. Epub 2021 Nov 16. Phytomedicine. 2022. PMID: 34856477
-
Chromium picolinate and chromium histidinate protects against renal dysfunction by modulation of NF-κB pathway in high-fat diet fed and Streptozotocin-induced diabetic rats.Nutr Metab (Lond). 2012 Apr 8;9:30. doi: 10.1186/1743-7075-9-30. Nutr Metab (Lond). 2012. PMID: 22483164 Free PMC article.
-
Inhibition of GSK-3β restores delayed gastric emptying in obesity-induced diabetic female mice.Am J Physiol Gastrointest Liver Physiol. 2020 Oct 1;319(4):G481-G493. doi: 10.1152/ajpgi.00227.2020. Epub 2020 Aug 19. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32812777 Free PMC article.
-
The potential therapeutic effects of Trifolium alexandrinum extract, hesperetin and quercetin against diabetic nephropathy via attenuation of oxidative stress, inflammation, GSK-3β and apoptosis in male rats.Chem Biol Interact. 2022 Jan 25;352:109781. doi: 10.1016/j.cbi.2021.109781. Epub 2021 Dec 17. Chem Biol Interact. 2022. PMID: 34922902
-
Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management.Acta Pharm Sin B. 2021 Sep;11(9):2749-2767. doi: 10.1016/j.apsb.2020.12.020. Epub 2021 Feb 2. Acta Pharm Sin B. 2021. PMID: 34589395 Free PMC article. Review.
Cited by
-
Astragaloside IV Relieves Mitochondrial Oxidative Stress Damage and Dysfunction in Diabetic Mice Endothelial Progenitor Cells by Regulating the GSK-3β/Nrf2 Axis.Appl Biochem Biotechnol. 2025 Jun;197(6):3964-3981. doi: 10.1007/s12010-025-05211-6. Epub 2025 Mar 25. Appl Biochem Biotechnol. 2025. PMID: 40131628
References
-
- Alves, R. , Suehiro, C. L. , Oliveira, F. G. D. , Frantz, E. D. C. , Medeiros, R. F. D. , Vieira, R. D. P. , Martins, M. D. A. , Lin, C. J. , Nobrega, A. C. L. D. , & Toledo‐Arruda, A. C. D. (2019). Aerobic exercise modulates cardiac NAD(P)H oxidase and the NRF2/KEAP1 pathway in a mouse model of chronic fructose consumption. Journal of Applied Physiology, 128(1), 59–69. 10.1152/japplphysiol.00201.2019 - DOI - PubMed
-
- Azhar, A. , Aamir, K. , Asad, F. , Kazi, H. A. , & Farooqui, M. U. (2019). Therapeutic effect of mango seed extract in diabetes mellitus. The Professional Medical Journal, 26(9), 1551–1556. 10.29309/TPMJ/2019.26.09.4023 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

