Immunostimulatory effects of marine algae extracts on in vitro antigen-presenting cell activation and in vivo immune cell recruitment
- PMID: 37823147
- PMCID: PMC10563723
- DOI: 10.1002/fsn3.3605
Immunostimulatory effects of marine algae extracts on in vitro antigen-presenting cell activation and in vivo immune cell recruitment
Abstract
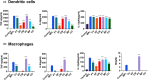
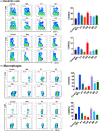
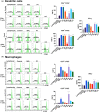
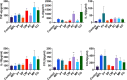
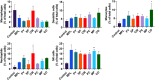
Marine algae are photosynthetic eukaryotic organisms that are widely used as sources of food, cosmetics, and drugs. However, their biological and immunological effects on immune cells have not been fully elucidated. To unravel their immunological activity and broaden their application, we generated antigen-presenting cells (APCs), including dendritic cells (DCs) and macrophages, from mouse bone marrow cells and treated them with six different marine algae extracts (MAEs). We evaluated cell viability, activation marker expression, and pro-inflammatory cytokine production by APCs after 2 days of MAE treatment. All six MAEs significantly induced cytokine production of APCs, among which Pyropia yezoensis (PY), Peyssonnelia caulifera (PC), and Meristotheca papulosa (MP) extracts exhibited the strongest effect. Cladophora wrightiana var. minor (CW) extract moderately upregulated cytokine levels but increased the expression of activation markers on DCs. Moreover, PY, PC, MP, Sargassum pectinifera (SP), and Caulerpa okamurae (CO) pre-treated APCs effectively stimulated T-cell proliferation and cytokine production. Furthermore, the mice injected with MAEs exhibited higher cytokine (TNF-α, IL-6, and IL-1β) production as well as enhanced innate immune cell recruitment capacities (DCs, monocytes, neutrophils, and natural killer cells) in the peritoneal cavity of the mice compared to those of the non-treated mice. Therefore, all MAEs exhibited immunostimulatory potential, with PY, PC, CW, and MP extracts being the most effective in stimulating immune responses and cell activation. To the best of our knowledge, this is the first study to determine the immunomodulatory activities of six MAEs both in vitro and in vivo.
Keywords: DCs; immunostimulatory effects; macrophages; marine algae extracts.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
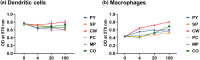
References
-
- Barzkar, N. , Khan, Z. , Jahromi, S. T. , Pourmozaffar, S. , Gozari, M. , & Nahavandi, R. (2021). A critical review on marine serine protease and its inhibitors: A new wave of drugs? International Journal of Biological Macromolecules, 170, 674–687. - PubMed
-
- Bhardwaj, M. , Mani, S. , Malarvizhi, R. , Sali, V. K. , & Vasanthi, H. R. (2021). Immunomodulatory activity of brown algae Turbinaria ornata derived sulfated polysaccharide on LPS induced systemic inflammation. Phytomedicine, 89, 153615. - PubMed
-
- Chaperot, L. , Chokri, M. , Jacob, M. , Drillat, P. , Garban, F. , Egelhofer, H. , Molens, J. P. , Sotto, J. J. , Bensa, J. C. , & Plumas, J. (2000). Differentiation of antigen‐presenting cells (dendritic cells and macrophages) for therapeutic application in patients with lymphoma. Leukemia, 14(9), 1667–1677. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

