Farnesoid X receptor prevents neutrophil extracellular traps via reduced sphingosine-1-phosphate in chronic kidney disease
- PMID: 37823198
- PMCID: PMC10894665
- DOI: 10.1152/ajprenal.00292.2023
Farnesoid X receptor prevents neutrophil extracellular traps via reduced sphingosine-1-phosphate in chronic kidney disease
Abstract
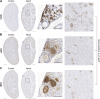
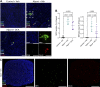
Farnesoid X receptor (FXR) activation reduces renal inflammation, but the underlying mechanisms remain elusive. Neutrophil extracellular traps (NETs) are webs of DNA formed when neutrophils undergo specialized programmed cell death (NETosis). The signaling lipid sphingosine-1-phosphate (S1P) stimulates NETosis via its receptor on neutrophils. Here, we identify FXR as a negative regulator of NETosis via repressing S1P signaling. We determined the effects of the FXR agonist obeticholic acid (OCA) in mouse models of adenosine phosphoribosyltransferase (APRT) deficiency and Alport syndrome, both genetic disorders that cause chronic kidney disease. Renal FXR activity is greatly reduced in both models, and FXR agonism reduces disease severity. Renal NETosis and sphingosine kinase 1 (Sphk1) expression are increased in diseased mice, and they are reduced by OCA in both models. Genetic deletion of FXR increases Sphk1 expression, and Sphk1 expression correlates with NETosis. Importantly, kidney S1P levels in Alport mice are two-fold higher than controls, and FXR agonism restores them back to baseline. Short-term inhibition of sphingosine synthesis in Alport mice with severe kidney disease reverses NETosis, establishing a causal relationship between S1P signaling and renal NETosis. Finally, extensive NETosis is present in human Alport kidney biopsies (six male, nine female), and NETosis severity correlates with clinical markers of kidney disease. This suggests the potential clinical relevance of the newly identified FXR-S1P-NETosis pathway. In summary, FXR agonism represses kidney Sphk1 expression. This inhibits renal S1P signaling, thereby reducing neutrophilic inflammation and NETosis.NEW & NOTEWORTHY Many preclinical studies have shown that the farnesoid X receptor (FXR) reduces renal inflammation, but the mechanism is poorly understood. This report identifies FXR as a novel regulator of neutrophilic inflammation and NETosis via the inhibition of sphingosine-1-phosphate signaling. Additionally, NETosis severity in human Alport kidney biopsies correlates with clinical markers of kidney disease. A better understanding of this signaling axis may lead to novel treatments that prevent renal inflammation and chronic kidney disease.
Keywords: Alport syndrome; NETosis; adenine diet; farnesoid X receptor; sphingosine-1-phosphate.
Conflict of interest statement
B.A.J. discloses a financial interest in AllazoHealth (New York, NY). None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures














References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

