Ghrelin Action in the PVH of Male Mice: Accessibility, Neuronal Targets, and CRH Neurons Activation
- PMID: 37823477
- PMCID: PMC11491828
- DOI: 10.1210/endocr/bqad154
Ghrelin Action in the PVH of Male Mice: Accessibility, Neuronal Targets, and CRH Neurons Activation
Abstract
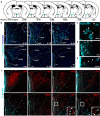
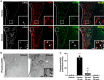
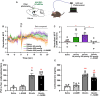
The hormone ghrelin displays several well-characterized functions, including some with pharmaceutical interest. The receptor for ghrelin, the growth hormone secretagogue receptor (GHSR), is expressed in the hypothalamic paraventricular nucleus (PVH), a critical hub for the integration of metabolic, neuroendocrine, autonomic, and behavioral functions. Here, we performed a neuroanatomical and functional characterization of the neuronal types mediating ghrelin actions in the PVH of male mice. We found that fluorescent ghrelin mainly labels PVH neurons immunoreactive for nitric oxide synthase 1 (NOS1), which catalyze the production of nitric oxide [NO]). Centrally injected ghrelin increases c-Fos in NOS1 PVH neurons and NOS1 phosphorylation in the PVH. We also found that a high dose of systemically injected ghrelin increases the ghrelin level in the cerebrospinal fluid and in the periventricular PVH, and induces c-Fos in NOS1 PVH neurons. Such a high dose of systemically injected ghrelin activates a subset of NOS1 PVH neurons, which do not express oxytocin, via an arcuate nucleus-independent mechanism. Finally, we found that pharmacological inhibition of NO production fully abrogates ghrelin-induced increase of calcium concentration in corticotropin-releasing hormone neurons of the PVH whereas it partially impairs ghrelin-induced increase of plasma glucocorticoid levels. Thus, plasma ghrelin can directly target a subset of NO-producing neurons of the PVH that is involved in ghrelin-induced activation of the hypothalamic-pituitary-adrenal neuroendocrine axis.
Keywords: CRF; GHSR; HPA axis; PVN; hypothalamus; stress.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974‐977. - PubMed
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656‐660. - PubMed
-
- Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083‐5086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

