Targeting phospholipid remodeling pathway improves insulin resistance in diabetic mouse models
- PMID: 37823674
- PMCID: PMC10575708
- DOI: 10.1096/fj.202301122RR
Targeting phospholipid remodeling pathway improves insulin resistance in diabetic mouse models
Abstract
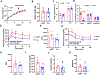
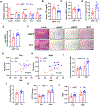
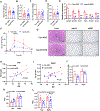
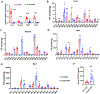
Previous studies have revealed that membrane phospholipid composition controlled by lysophosphatidylcholine acyltransferase 3 (LPCAT3) is involved in the development of insulin resistance in type 2 diabetes. In this study, we aimed to investigate the therapeutic potential of targeting Lpcat3 in the treatment of insulin resistance in diabetic mouse models. Lpcat3 expression was suppressed in the whole body by antisense oligonucleotides (ASO) injection or in the liver by adeno-associated virus (AAV)-encoded Cre in high-fat diet (HFD)-induced and genetic ob/ob type 2 diabetic mouse models. Glucose tolerance test (GTT), insulin tolerance test (ITT), fasting blood glucose, and insulin levels were used to assess insulin sensitivity. Lipid levels in the liver and serum were measured. The expression of genes involved in de novo lipogenesis was analyzed by real-time RT-PCR. Metabolic rates were measured by indirect calorimetry using the Comprehensive Lab Animal Monitoring System (CLAMS). Our data demonstrate that acute knockout of hepatic Lpcat3 by AAV-Cre improves both hyperglycemia and hypertriglyceridemia in HFD-fed mice. Similarly, whole-body ablation of Lpcat3 by ASO administration improves obesity and insulin resistance in both HFD-fed and ob/ob mice. These findings demonstrate that targeting LPCAT3 could be a novel therapy for insulin resistance.
Keywords: LPCAT3; insulin resistance; obesity; phospholipid remodeling; type 2 diabetes.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Figures



References
-
- Magliano DJ, and Boyko EJ (2021) In IDF DIABETES ATLAS, Brussels
-
- Zheng Y, Ley SH, and Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14, 88–98 - PubMed
-
- Brown MS, and Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7, 95–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

