Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans
- PMID: 37823690
- PMCID: PMC10572082
- DOI: 10.1080/19420862.2023.2261509
Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans
Abstract
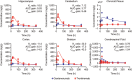
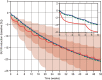
There are few treatments that slow neurodegeneration in Alzheimer's disease (AD), and while therapeutic antibodies are being investigated in clinical trials for AD treatment, their access to the central nervous system is restricted by the blood-brain barrier. This study investigates a bispecific modular fusion protein composed of gantenerumab, a fully human monoclonal anti- amyloid-beta (Aβ) antibody under investigation for AD treatment, with a human transferrin receptor 1-directed Brainshuttle™ module (trontinemab; RG6102, INN trontinemab). In vitro, trontinemab showed a similar binding affinity to fibrillar Aβ40 and Aβ plaques in human AD brain sections to gantenerumab. A single intravenous administration of trontinemab (10 mg/kg) or gantenerumab (20 mg/kg) to non-human primates (NHPs, Macaca fascicularis), was well tolerated in both groups. Immunohistochemistry indicated increased trontinemab uptake into the brain endothelial cell layer and parenchyma, and more homogeneous distribution, compared with gantenerumab. Brain and plasma pharmacokinetic (PK) parameters for trontinemab were estimated by nonlinear mixed-effects modeling with correction for tissue residual blood, indicating a 4-18-fold increase in brain exposure. A previously developed clinical PK/pharmacodynamic model of gantenerumab was adapted to include a brain compartment as a driver of plaque removal and linked to the allometrically scaled above model from NHP. The new brain exposure-based model was used to predict trontinemab dosing regimens for effective amyloid reduction. Simulations from these models were used to inform dosing of trontinemab in the first-in-human clinical trial.
Keywords: Alzheimer’s disease; Brainshuttle™; blood–brain barrier; gantenerumab; monoclonal antibody; pharmacokinetics.
Conflict of interest statement
HPG, VS, POF, KB, CH, SR, SS, RN, NJ were employees and shareholders of F. Hoffmann-La Roche Ltd at the time the work was completed.
MS, SIJ, PR, TS, UG, MH, AZ, JN were employees of Roche Diagnostics GmbH and shareholders of F. Hoffmann-La Roche Ltd at the time the work was completed.
Figures



References
-
- US Food and Drug Administration (FDA) . FDA’s decision to approve new treatment for Alzheimer’s disease [press release]; 2021. June 7 [accessed 2023 June 28]. https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-....
-
- US Food and Drug Administration (FDA) . FDA grants accelerated approval for Alzheimer’s disease treatment [press release]; 2023. January 6 [accessed 2023 June 28]. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat....
-
- Aging NNIo . NIA statement on report of lecanemab reducing cognitive decline in Alzheimer’s clinical trial; 2023. [accessed 2023 March 16]. https://www.nia.nih.gov/news/nia-statement-report-lecanemab-reducing-cog...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
