Immune signatures of checkpoint inhibitor-induced autoimmunity-A focus on neurotoxicity
- PMID: 37823709
- PMCID: PMC10836772
- DOI: 10.1093/neuonc/noad198
Immune signatures of checkpoint inhibitor-induced autoimmunity-A focus on neurotoxicity
Abstract
Background: Neurologic immune-related adverse events (irAE-n) are rare but severe toxicities of immune checkpoint inhibitor (ICI) treatment. To overcome diagnostic and therapeutic challenges, a better mechanistic understanding of irAE-n is paramount.
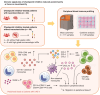
Methods: In this observational cohort study, we collected serum and peripheral blood samples from 34 consecutive cancer patients with irAE-n (during acute illness) and 49 cancer control patients without irAE-n (pre- and on-ICI treatment, n = 44 without high-grade irAEs, n = 5 with high-grade nonneurologic irAEs). Patients received either anti-programmed cell death protein (PD)-1 or anti-PD ligand-1 monotherapy or anti-PD-1/anti-cytotoxic T-lymphocyte-associated protein-4 combination therapy. Most common cancers were melanoma, lung cancer, and hepatocellular carcinoma. Peripheral blood immune profiling was performed using 48-marker single-cell mass cytometry and a multiplex cytokine assay.
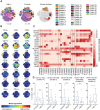
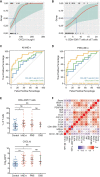
Results: During acute illness, patients with irAE-n presented higher frequencies of cluster of differentiation (CD)8+ effector memory type (EM-)1 and central memory (CM) T cells compared to controls without irAEs. Multiorgan immunotoxicities (neurologic + nonneurologic) were associated with higher CD8+ EM1 T cell counts. While there were no B cell changes in the overall cohort, we detected a marked decrease of IgD- CD11c+ CD21low and IgD- CD24+ CD21high B cells in a subgroup of patients with autoantibody-positive irAE-n. We further identified signatures indicative of enhanced chemotaxis and inflammation in irAE-n patients and discovered C-X-C motif chemokine ligand (CXCL)10 as a promising marker to diagnose high-grade immunotoxicities such as irAE-n.
Conclusions: We demonstrate profound and partly subgroup-specific immune cell dysregulation in irAE-n patients, which may guide future biomarker development and targeted treatment approaches.
Keywords: immune checkpoint inhibitors; immune-related adverse events; immunotherapy; mass cytometry; neurotoxicity.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
L.M.J., R.M., C.U., P.K., N.F., S.F., D.K., C.S., L.G.R., S.K.M., F.T.N., and M.E. have declared that no conflict of interest exists related to the submitted work. A.R.S. and H.E.M. are listed as inventors on patents relating to mass cytometry reagents; H.E.M. receives royalties from Standard BioTools. L.H. declares research support from Therakos; speakers and advisory board honoraria from 4SC, Amgen, BiomeDx, Bristol Myers Squibb, Curevac, Merck, Merck Sharp & Dohme, Myoncare, Novartis, Pierre Fabre, Sanofi, SUN, and Roche. L.H. further holds patents described in publications nos. (1) WO/2001/052874; PCT/EP2001/000363, (2) WO/2003/093419; PCT/US2003/013350, and (3) WO/2019/219705; PCT/EP2019/062378. W.B. and P.H. have received lecture fees from Bristol-Meyers-Squibb (W.B.) and/or NOGGO e.V. (W.B. and P.H.), all unrelated to this work. S.K. has received lecture fees from the Nationale Gesundheits-Akademie GmbH, outside of the submitted work.
Figures



References
-
- Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. - PubMed
-
- Marini A, Bernardini A, Gigli GL, et al. . Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. 2021;96(16):754–766. - PubMed
-
- Mikami T, Liaw B, Asada M, et al. . Neuroimmunological adverse events associated with immune checkpoint inhibitor: a retrospective, pharmacovigilance study using FAERS database. J Neurooncol. 2021;152(1):135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

