Combined Immunotherapy Improves Outcome for Replication-Repair-Deficient (RRD) High-Grade Glioma Failing Anti-PD-1 Monotherapy: A Report from the International RRD Consortium
- PMID: 37823831
- PMCID: PMC10850948
- DOI: 10.1158/2159-8290.CD-23-0559
Combined Immunotherapy Improves Outcome for Replication-Repair-Deficient (RRD) High-Grade Glioma Failing Anti-PD-1 Monotherapy: A Report from the International RRD Consortium
Abstract
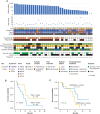
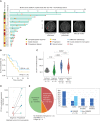
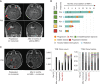
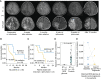
Immune checkpoint inhibition (ICI) is effective for replication-repair-deficient, high-grade gliomas (RRD-HGG). The clinical/biological impact of immune-directed approaches after failing ICI monotherapy is unknown. We performed an international study on 75 patients treated with anti-PD-1; 20 are progression free (median follow-up, 3.7 years). After second progression/recurrence (n = 55), continuing ICI-based salvage prolonged survival to 11.6 months (n = 38; P < 0.001), particularly for those with extreme mutation burden (P = 0.03). Delayed, sustained responses were observed, associated with changes in mutational spectra and the immune microenvironment. Response to reirradiation was explained by an absence of deleterious postradiation indel signatures (ID8). CTLA4 expression increased over time, and subsequent CTLA4 inhibition resulted in response/stable disease in 75%. RAS-MAPK-pathway inhibition led to the reinvigoration of peripheral immune and radiologic responses. Local (flare) and systemic immune adverse events were frequent (biallelic mismatch-repair deficiency > Lynch syndrome). We provide a mechanistic rationale for the sustained benefit in RRD-HGG from immune-directed/synergistic salvage therapies. Future approaches need to be tailored to patient and tumor biology.
Significance: Hypermutant RRD-HGG are susceptible to checkpoint inhibitors beyond initial progression, leading to improved survival when reirradiation and synergistic immune/targeted agents are added. This is driven by their unique biological and immune properties, which evolve over time. Future research should focus on combinatorial regimens that increase patient survival while limiting immune toxicity. This article is featured in Selected Articles from This Issue, p. 201.
©2023 American Association for Cancer Research.
Figures

References
-
- Tabori U, Hansford JR, Achatz MI, Kratz CP, Plon SE, Frebourg T, et al. . Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood. Clin Cancer Res 2017;23:e32–e7. - PubMed
-
- Shlien A, Campbell BB, de Borja R, Alexandrov LB, Merico D, Wedge D, et al. . Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet 2015;47:257–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

