Green Oxidation of Heterocyclic Ketones with Oxone in Water
- PMID: 37823876
- PMCID: PMC10629238
- DOI: 10.1021/acs.joc.3c01513
Green Oxidation of Heterocyclic Ketones with Oxone in Water
Abstract
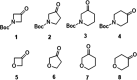
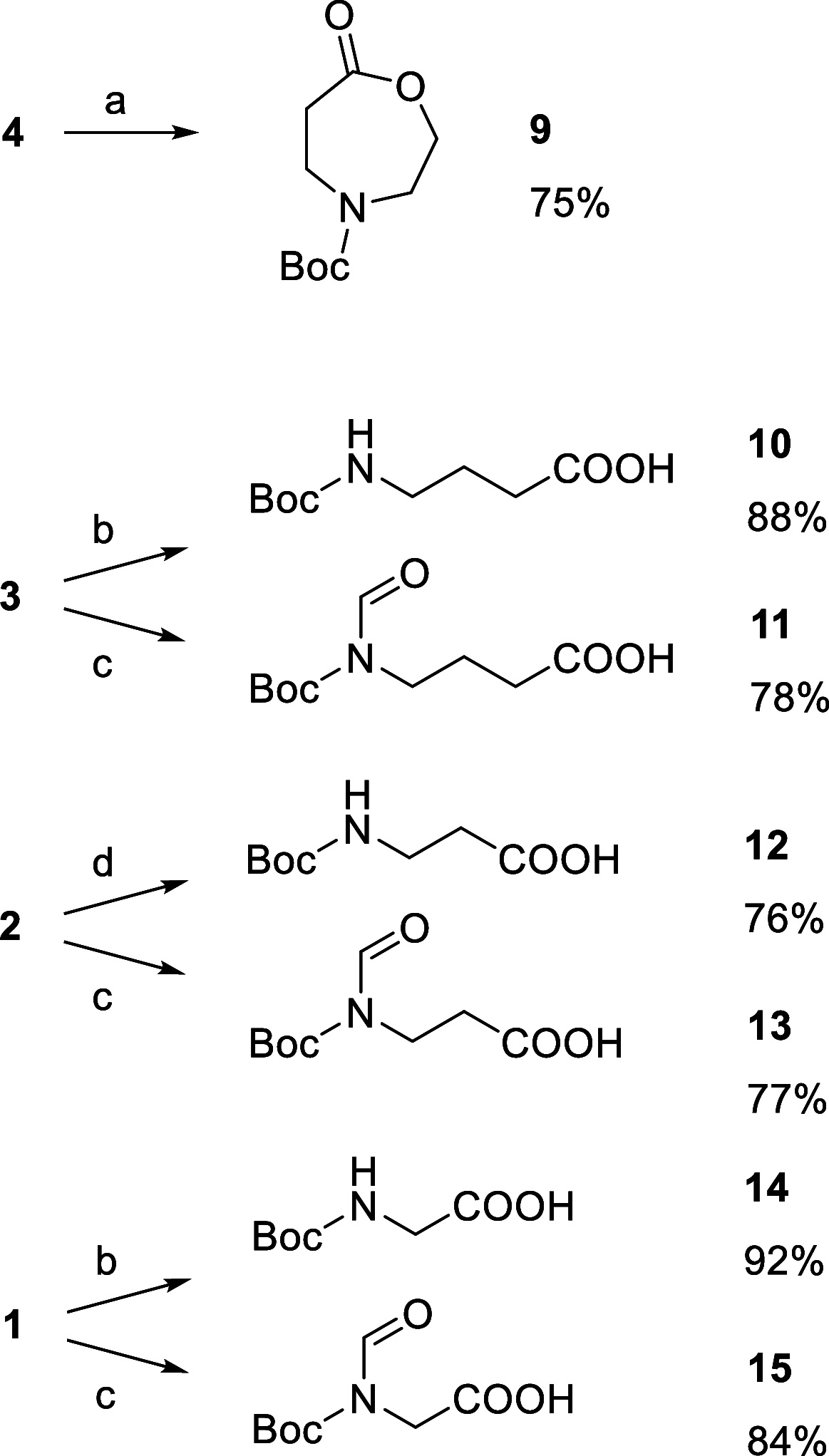
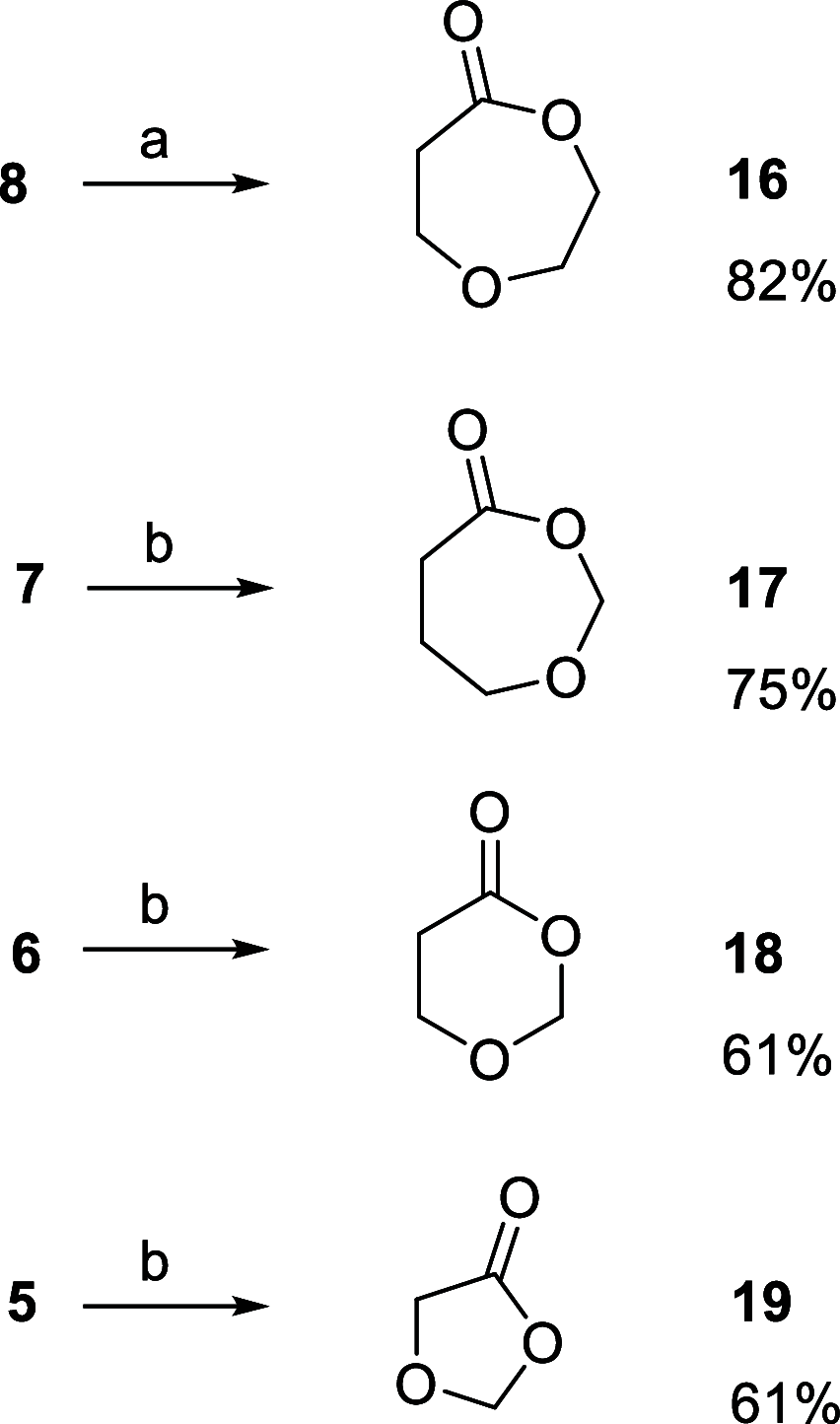
The recently reported efficient conversion of cyclic ketones to lactones by Oxone in neutral buffered water is extended to heterocyclic ketones, namely, cyclic N-Boc azaketones and oxoethers with the aim of obtaining N-protected azalactones and their analogues with oxygen in place of nitrogen. N-Boc-4-piperidinone and all the cyclic oxoethers were successfully oxidized to lactones, while the azacyclic ketones with nitrogen α-positioned to carbonyl were univocally transformed into N-Boc-ω-amino acids and N-Boc-N-formyl-ω-amino acids operating in alkaline water and DMF, respectively.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sharley J. S.; Gambacorta G.; Collado Perez A. M.; Ferri E. E.; Miranda A. F.; Fernandez I. F.; Quesada J. S.; Baxendale I. R. A simple one-pot oxidation protocol for the synthesis of dehydrohedione from Hedione. Tetrahedron 2022, 126, 133068.10.1016/j.tet.2022.133068. - DOI
- Feng Y.; Chang B.; Ren Y.; Zhao F.; Wang K. H.; Wang J.; Huang D.; Lv X.; Hu Y. Synthesis of trifluoromethyl pyrrolopyrazole derivatives via [3 + 2] cycloaddition of trifluoromethyl N-acylhydrazones or trifluoroacetohydrazonoyl bromides with maleimides. Tetrahedron 2023, 136, 133353.10.1016/j.tet.2023.133353. - DOI
- Bolchi C.; Valoti E.; Straniero V.; Ruggeri P.; Pallavicini M. From 2-aminomethyl-1,4-benzodioxane enantiomers to unichiral 2-cyano- and 2-carbonyl-substituted benzodioxanes via dichloroamine. J. Org. Chem. 2014, 79, 6732.10.1021/jo500964y. - DOI - PubMed
- Pallavicini M.; Bolchi C.; Fumagalli L.; Piccolo O.; Valoti E. Highly efficient racemization of a key intermediate of the antibiotic moxifloxacin. Tetrahedron Asymmetry 2011, 22, 379.10.1016/j.tetasy.2011.02.007. - DOI
- Kraemer Y.; Bergman E. N.; Togni A.; Pitts C. R. Oxidative fluorination of heteroatoms enabled by trichloroisocyanuric acid and potassium fluoride. Angew. Chem., Int. Ed. 2022, 61, e20220508810.1002/anie.202205088. - DOI - PMC - PubMed
-
- Dorado V.; Herrerias C. I.; Fraile J. M. Simple metal-free oxidative cleavage of 1,2-diols. Tetrahedron 2023, 139, 133450.10.1016/j.tet.2023.133450. - DOI
- Zhang M. Z.; Wang P.; Liu H. Y.; Wang D.; Deng Y.; Bai Y. D.; Luo F.; Wu W. Y.; Chen T. Metal-catalyst-free one-pot aqueous synthesis of trans-1,2-diols from electron-deficient α,β-unsaturated amides via epoxidation using oxone as a dual role reagent. ChemSusChem 2023, 16, e20230058310.1002/cssc.202300583. - DOI - PubMed
- Alvi S.; Jayant V.; Ali R. Applications of oxone in organic synthesis: an emerging green reagent of modern era. ChemistrySelect 2022, 7, e20220070410.1002/slct.202200704. - DOI
-
- Xu J.; Liang L.; Zheng H.; Chi Y. R.; Tong R. Green oxidation of indoles using halide catalysis. Nat. Commun. 2019, 10, 4754.10.1038/s41467-019-12768-4. - DOI - PMC - PubMed
- Sathish M.; Sakla A. P.; Nachtigall F. M.; Santos L. S.; Shankaraiah N. TCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline. RSC Adv. 2021, 11, 16537.10.1039/D1RA02381K. - DOI - PMC - PubMed
-
- Blödorn G. B.; Duarte L. F. B.; Roehrs J. A.; Silva M. S.; Neto J. S. S.; Alves D. Trichloroisocyanuric acid (TCCA): a suitable reagent for the synthesis of selanyl-benzo[b]chalcogenophenes. Eur. J. Org. Chem. 2022, 2022, e20220077510.1002/ejoc.202200775. - DOI

