Early and Empirical High-Dose Cryoprecipitate for Hemorrhage After Traumatic Injury: The CRYOSTAT-2 Randomized Clinical Trial
- PMID: 37824155
- PMCID: PMC10570921
- DOI: 10.1001/jama.2023.21019
Early and Empirical High-Dose Cryoprecipitate for Hemorrhage After Traumatic Injury: The CRYOSTAT-2 Randomized Clinical Trial
Abstract
Importance: Critical bleeding is associated with a high mortality rate in patients with trauma. Hemorrhage is exacerbated by a complex derangement of coagulation, including an acute fibrinogen deficiency. Management is fibrinogen replacement with cryoprecipitate transfusions or fibrinogen concentrate, usually administered relatively late during hemorrhage.
Objective: To assess whether survival could be improved by administering an early and empirical high dose of cryoprecipitate to all patients with trauma and bleeding that required activation of a major hemorrhage protocol.
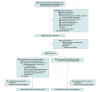
Design, setting, and participants: CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study. Patients were enrolled at 26 UK and US major trauma centers from August 2017 to November 2021. Eligible patients were injured adults requiring activation of the hospital's major hemorrhage protocol with evidence of active hemorrhage, systolic blood pressure less than 90 mm Hg at any time, and receiving at least 1 U of a blood component transfusion.
Intervention: Patients were randomly assigned (in a 1:1 ratio) to receive standard care, which was the local major hemorrhage protocol (reviewed for guideline adherence), or cryoprecipitate, in which 3 pools of cryoprecipitate (6-g fibrinogen equivalent) were to be administered in addition to standard care within 90 minutes of randomization and 3 hours of injury.
Main outcomes and measures: The primary outcome was all-cause mortality at 28 days in the intention-to-treat population.
Results: Among 1604 eligible patients, 799 were randomized to the cryoprecipitate group and 805 to the standard care group. Missing primary outcome data occurred in 73 patients (principally due to withdrawal of consent) and 1531 (95%) were included in the primary analysis population. The median (IQR) age of participants was 39 (26-55) years, 1251 (79%) were men, median (IQR) Injury Severity Score was 29 (18-43), 36% had penetrating injury, and 33% had systolic blood pressure less than 90 mm Hg at hospital arrival. All-cause 28-day mortality in the intention-to-treat population was 26.1% in the standard care group vs 25.3% in the cryoprecipitate group (odds ratio, 0.96 [95% CI, 0.75-1.23]; P = .74). There was no difference in safety outcomes or incidence of thrombotic events in the standard care vs cryoprecipitate group (12.9% vs 12.7%).
Conclusions and relevance: Among patients with trauma and bleeding who required activation of a major hemorrhage protocol, the addition of early and empirical high-dose cryoprecipitate to standard care did not improve all cause 28-day mortality.
Trial registration: ClinicalTrials.gov Identifier: NCT04704869; ISRCTN Identifier: ISRCTN14998314.
Conflict of interest statement
Figures


Comment in
-
Contemporary Adjuncts to Hemorrhage Control.JAMA. 2023 Nov 21;330(19):1849-1851. doi: 10.1001/jama.2023.16135. JAMA. 2023. PMID: 37824165 No abstract available.
-
Trauma-induced coagulopathy, could cryoprecipitates improve outcomes?CJEM. 2024 Jul;26(7):458-459. doi: 10.1007/s43678-024-00704-5. Epub 2024 Apr 30. CJEM. 2024. PMID: 38689201 No abstract available.

