Loss of ATOH1 in Pit Cell Drives Stemness and Progression of Gastric Adenocarcinoma by Activating AKT/mTOR Signaling through GAS1
- PMID: 37824217
- PMCID: PMC10646280
- DOI: 10.1002/advs.202301977
Loss of ATOH1 in Pit Cell Drives Stemness and Progression of Gastric Adenocarcinoma by Activating AKT/mTOR Signaling through GAS1
Abstract
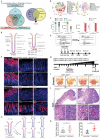
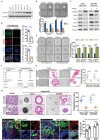
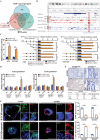
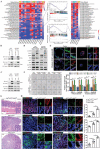
Gastric cancer stem cells (GCSCs) are self-renewing tumor cells that govern chemoresistance in gastric adenocarcinoma (GAC), whereas their regulatory mechanisms remain elusive. Here, the study aims to elucidate the role of ATOH1 in the maintenance of GCSCs. The preclinical model and GAC sample analysis indicate that ATOH1 deficiency is correlated with poor GAC prognosis and chemoresistance. ScRNA-seq reveals that ATOH1 is downregulated in the pit cells of GAC compared with those in paracarcinoma samples. Lineage tracing reveals that Atoh1 deletion strongly confers pit cell stemness. ATOH1 depletion significantly accelerates cancer stemness and chemoresistance in Tff1-CreERT2; Rosa26Tdtomato and Tff1-CreERT2; Apcfl/fl ; p53fl/fl (TcPP) mouse models and organoids. ATOH1 deficiency downregulates growth arrest-specific protein 1 (GAS1) by suppressing GAS1 promoter transcription. GAS1 forms a complex with RET, which inhibits Tyr1062 phosphorylation, and consequently activates the RET/AKT/mTOR signaling pathway by ATOH1 deficiency. Combining chemotherapy with drugs targeting AKT/mTOR signaling can overcome ATOH1 deficiency-induced chemoresistance. Moreover, it is confirmed that abnormal DNA hypermethylation induces ATOH1 deficiency. Taken together, the results demonstrate that ATOH1 loss promotes cancer stemness through the ATOH1/GAS1/RET/AKT/mTOR signaling pathway in GAC, thus providing a potential therapeutic strategy for AKT/mTOR inhibitors in GAC patients with ATOH1 deficiency.
Keywords: ATOH1; GAS1; gastric adenocarcinoma; mouse model; stemness.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Tan S. H., Swathi Y., Tan S., Goh J., Seishima R., Murakami K., Oshima M., Tsuji T., Phuah P., Tan L. T., Wong E., Fatehullah A., Sheng T., Ho S. W. T., Grabsch H. I., Srivastava S., Teh M., Denil S. L. I. J., Mustafah S., Tan P., Shabbir A., So J., Yeoh K. G., Barker N., Nature 2020, 578, 437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023QN01010108/Youth Research Planning Project of Fujian Province Health Commission Science and Technology
- 81871899/National Natural Science Foundation of China
- [2021]76/Construction Project of Fujian Province Minimally Invasive Medical Center
- 2022XH021/Excellent Young Scholars Cultivation Project of Fujian Medical University Union Hospital
- 2020QH2028/Scientific Research Foundation of Graduate School of Harbin Medical University: Sino Russian Special Fund
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
