Structural and functional specializations of human fast-spiking neurons support fast cortical signaling
- PMID: 37824618
- PMCID: PMC10569701
- DOI: 10.1126/sciadv.adf0708
Structural and functional specializations of human fast-spiking neurons support fast cortical signaling
Abstract
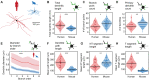
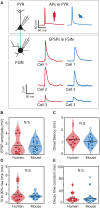
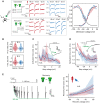
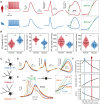
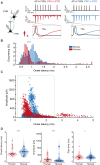
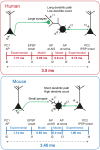
Fast-spiking interneurons (FSINs) provide fast inhibition that synchronizes neuronal activity and is critical for cognitive function. Fast synchronization frequencies are evolutionary conserved in the expanded human neocortex despite larger neuron-to-neuron distances that challenge fast input-output transfer functions of FSINs. Here, we test in human neurons from neurosurgery tissue, which mechanistic specializations of human FSINs explain their fast-signaling properties in human cortex. With morphological reconstructions, multipatch recordings, and biophysical modeling, we find that despite threefold longer dendritic path, human FSINs maintain fast inhibition between connected pyramidal neurons through several mechanisms: stronger synapse strength of excitatory inputs, larger dendrite diameter with reduced complexity, faster AP initiation, and faster and larger inhibitory output, while Na+ current activation/inactivation properties are similar. These adaptations underlie short input-output delays in fast inhibition of human pyramidal neurons through FSINs, explaining how cortical synchronization frequencies are conserved despite expanded and sparse network topology of human cortex.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

