Tobacco toxins induce osteoporosis through ferroptosis
- PMID: 37826866
- PMCID: PMC10571034
- DOI: 10.1016/j.redox.2023.102922
Tobacco toxins induce osteoporosis through ferroptosis
Abstract
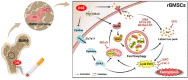
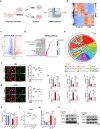
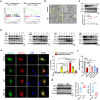
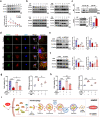
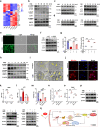
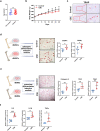
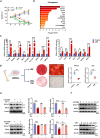
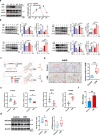
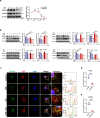
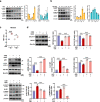
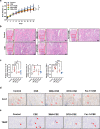
Clinical epidemiological studies have confirmed that tobacco smoking disrupts bone homeostasis and is an independent risk factor for the development of osteoporosis. The low viability and inferior osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) are important etiologies of osteoporosis. However, few basic studies have elucidated the specific mechanisms that tobacco toxins devastated BMSCs and consequently induced or exacerbated osteoporosis. Herein, our clinical data showed the bone mineral density (BMD) values of femoral neck in smokers were significantly lower than non-smokers, meanwhile cigarette smoke extract (CSE) exposure led to a significant decrease of BMD in rats and dysfunction of rat BMSCs (rBMSCs). Transcriptomic analysis and phenotype experiments suggested that the ferroptosis pathway was significantly activated in CSE-treated rBMSCs. Accumulated intracellular reactive oxygen species activated AMPK signaling, furtherly promoted NCOA4-mediated ferritin-selective autophagic processes, increased labial iron pool and lipid peroxidation deposition, and ultimately led to ferroptosis in rBMSCs. Importantly, in vivo utilization of ferroptosis and ferritinophagy inhibitors significantly alleviated BMD loss in CSE-exposed rats. Our study innovatively reveals the key mechanism of smoking-related osteoporosis, and provides a possible route targeting on the perspective of BMSC ferroptosis for future prevention and treatment of smoking-related bone homeostasis imbalance.
Keywords: Bone marrow mesenchymal stem cells; Cigarette smoke extract; Ferritinophagy; Ferroptosis; Osteoporosis.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing financial interests.
Figures



References
-
- World Health Organization . WHO; 2022. Tobacco.https://www.who.int/news-room/fact-sheets/detail/tobacco
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

