Mapping mesoscale connectivity within the human hippocampus
- PMID: 37827206
- PMCID: PMC10623761
- DOI: 10.1016/j.neuroimage.2023.120406
Mapping mesoscale connectivity within the human hippocampus
Abstract
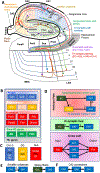
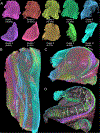
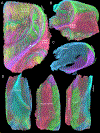
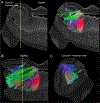
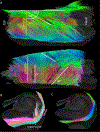
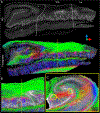
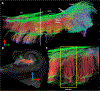
The connectivity of the hippocampus is essential to its functions. To gain a whole system view of intrahippocampal connectivity, ex vivo mesoscale (100 μm isotropic resolution) multi-shell diffusion MRI (11.7T) and tractography were performed on entire post-mortem human right hippocampi. Volumetric measurements indicated that the head region was largest followed by the body and tail regions. A unique anatomical organization in the head region reflected a complex organization of the granule cell layer (GCL) of the dentate gyrus. Tractography revealed the volumetric distribution of the perforant path, including both the tri-synaptic and temporoammonic pathways, as well as other well-established canonical connections, such as Schaffer collaterals. Visualization of the perforant path provided a means to verify the borders between the pro-subiculum and CA1, as well as between CA1/CA2. A specific angularity of different layers of fibers in the alveus was evident across the whole sample and allowed a separation of afferent and efferent connections based on their origin (i.e. entorhinal cortex) or destination (i.e. fimbria) using a cluster analysis of streamlines. Non-canonical translamellar connections running along the anterior-posterior axis were also discerned in the hilus. In line with "dentations" of the GCL, mossy fibers were bunching together in the sagittal plane revealing a unique lamellar organization and connections between these. In the head region, mossy fibers projected to the origin of the fimbria, which was distinct from the body and tail region. Mesoscale tractography provides an unprecedented systems view of intrahippocampal connections that underpin cognitive and emotional processing.
Keywords: Connectivity; Dentate Gyrus; Hippocampus; Mesoscale; Mossy Fibers; Perforant path; Tractography; Tri-synaptic circuit.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no personal financial or institutional interest in the results described in this article.
Figures
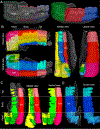




References
-
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA, 2018. Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc. Natl. Acad. Sci. USA 115, 4252–4257. - PMC - PubMed
-
- Amaral DG, 1978. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J. Comp. Neurol 182, 851–914. - PubMed
-
- Amaral DG, 1993. Emerging principles of intrinsic hippocampal organization. Curr. Opin. Neurobiol 3, 225–229. - PubMed
-
- Amaral DG, Witter MP, 1989. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

