Central Nervous System Distribution of Panobinostat in Preclinical Models to Guide Dosing for Pediatric Brain Tumors
- PMID: 37827699
- PMCID: PMC10658912
- DOI: 10.1124/jpet.123.001826
Central Nervous System Distribution of Panobinostat in Preclinical Models to Guide Dosing for Pediatric Brain Tumors
Abstract
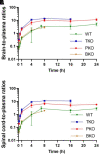
Achieving adequate exposure of the free therapeutic agent at the target is a critical determinant of efficacious chemotherapy. With this in mind, a major challenge in developing therapies for central nervous system (CNS) tumors is to overcome barriers to delivery, including the blood-brain barrier (BBB). Panobinostat is a nonselective pan-histone deacetylase inhibitor that is being tested in preclinical and clinical studies, including for the treatment of pediatric medulloblastoma, which has a propensity for leptomeningeal spread and diffuse midline glioma, which can infiltrate into supratentorial brain regions. In this study, we examined the rate, extent, and spatial heterogeneity of panobinostat CNS distribution in mice. Transporter-deficient mouse studies show that panobinostat is a dual substrate of P-glycoprotein (P-gp) and breast cancer resistant protein (Bcrp), which are major efflux transporters expressed at the BBB. The CNS delivery of panobinostat was moderately limited by P-gp and Bcrp, and the unbound tissue-to-plasma partition coefficient of panobinostat was 0.32 and 0.21 in the brain and spinal cord in wild-type mice. In addition, following intravenous administration, panobinostat demonstrated heterogeneous distribution among brain regions, indicating that its efficacy would be influenced by tumor location or the presence and extent of leptomeningeal spread. Simulation using a compartmental BBB model suggests inadequate exposure of free panobinostat in the brain following a recommended oral dosing regimen in patients. Therefore, alternative approaches to CNS delivery may be necessary to have adequate exposure of free panobinostat for the treatment of a broad range of pediatric brain tumors. SIGNIFICANCE STATEMENT: This study shows that the central nervous system (CNS) penetration of panobinostat is limited by P-gp and Bcrp, and its efficacy may be limited by inadequate distribution to the tumor. Panobinostat has heterogeneous distribution into various brain regions, indicating that its efficacy might depend on the anatomical location of the tumors. These distributional parameters in the mouse CNS can inform both preclinical and clinical trial study design and may guide treatment for these devastating brain tumors in children.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures








References
-
- Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280:233–241. - PubMed
-
- Bailer AJ (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16:303–309. - PubMed
-
- Buckner JC, Brown PD, O’Neill BP, Meyer FB, Wetmore CJ, Uhm JH (2007) Central nervous system tumors. Mayo Clin Proc 82:1271–1286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

