Comparison of 2 Methods for Estimating Multiple Sclerosis-Related Mortality
- PMID: 37827849
- PMCID: PMC10791051
- DOI: 10.1212/WNL.0000000000207925
Comparison of 2 Methods for Estimating Multiple Sclerosis-Related Mortality
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Determining whether multiple sclerosis (MS) causes death is challenging. Our objective was to contrast 2 frameworks to estimate probabilities of death attributed to MS (PMS) and other causes (Pother): the cause-specific framework (CSF), which requires the causes of death, and the excess mortality framework (EMF), which does not.
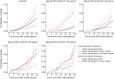
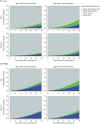
Methods: We used data from the Observatoire Français de la Sclérose en Plaques (OFSEP, n = 37,524) and from a comparative subset where causes of death were available (4,004 women with relapsing-onset MS [R-MS]). In CSF, the probabilities were estimated using the Aalen-Johansen method. In EMF, they were estimated from the excess mortality hazard, which is the additional mortality among patients with MS as compared with the expected mortality in the matched general population. PMS values were estimated at 30 years of follow-up, (1) with both frameworks in the comparative subset, by age group at onset, and (2) with EMF only in the OFSEP population, by initial phenotype, sex, and age at onset.
Results: In the comparative subset, the estimated 30-year PMS values were greater using EMF than CSF: 10.9% (95% CI 8.3-13.6) vs 8.7% (6.4-11.8) among the youngest and 20.4% (11.3-29.5) vs 16.2% (8.7-30.2) for the oldest groups, respectively. In the CSF, probabilities of death from unknown causes ranged from 1.5% (0.7-3.0) to 6.4% (2.5-16.4), and even after their reallocation, PMS values remained lower with CSF than with EMF. The estimated probabilities of being alive were close using the 2 frameworks, and the estimated POther (EMF vs CSF) was 2.6% (2.5-2.6) vs 2.1% (1.2-3.9) and 18.1% (16.9-19.3) vs 26.4% (16.5-42.2), respectively, for the youngest and oldest groups. In the OFSEP population, the estimated 30-year PMS values ranged from 7.5% (6.4-8.7) to 24.0% (19.1-28.9) in patients with R-MS and from 25.4% (21.1-29.7) to 36.8% (28.3-45.3) in primary progressive patients, depending on sex and age.
Discussion: EMF has the great advantage of not requiring death certificates, their quality being less than optimal. Conceptually, it also seems more relevant because it avoids having to state, for each individual, whether death was directly or indirectly caused by MS or whether it would have occurred anyway, which is especially difficult in such chronic diseases.
Conflict of interest statement
F. Rollot, Z. Uhry, and E. Dantony report no disclosures. S. Vukusic reports consulting and lecture fees, travel grants, and research support from Biogen, BMS-Celgene, Janssen, Merck, Novartis, Roche, Sandoz, Sanofi-Genzyme, and Teva Pharma. Nothing related to the contents of the present work. M. Debouverie reports no disclosures. E. Le Page reports talking for: Biogen, Genzyme, Merck-Serono, Novartis, Roche, Teva, Alexion; Advisory Boards: Biogen, Genzyme, Novartis, Merck, Jansen, Merck; Research grants: ARSEP, Ligue, TEVA. J. Ciron reports consulting and lecturing fees, travel grants from Biogen, Novartis, Merck, Teva, Sanofi-Genzyme, Roche, Celgene, and Alexion. A. Ruet reports grant, personal fees, and non-financial support from Novartis; grants, personal fees, and non-financial support from Biogen; grant personal fees and non-financial support from Roche; grant personal fees and non-financial support from Genzyme; grant personal fees from Merck; personal fees and non-financial support from Alexion; personal fees from Horizon Therapeutics, all of them not related to the submitted study. J. De Sèze reports no disclosures. H. Zéphir reports consulting or lectures, and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis, and Bayer, as well as research support from Teva and Roche, and academic research grants from the Académie de Médecine, LFSEP, FHU Imminent and ARSEP Foundation. P. Labauge received honoraria for consulting from Biogen, Merck, Sanofi, and Novartis. G. Defer has received personal compensation for the scientific advisory board for Biogen, Novartis, Genzyme, Merck Serono, Roche, and Teva. He has received speaker honoraria and travel grants from Merck Serono, Biogen, Novartis, Roche, Genzyme, and Teva. His institution has received research support in his department from Merck Serono, Biogen, Novartis, and Genzyme. C. Lebrun-Frenay reports no disclosures. T. Moreau reports fees as scientific adviser from Biogen, Medday, Novartis, Genzyme, Sanofi and Roche. D.A. Laplaud reports consulting fees from Alexion, Biogen, BMS, Merck, Novartis, Sanofi, and Roche. E. Berger reports honoraria and consulting fees from Novartis, Sanofi Aventis, Biogen, Genzyme, Roche, Teva Pharma, and Merck. P. Clavelou reports consulting and lecture fees or travel grants from Biogen, Janssen, Medday, Merck, Novartis, Roche, Sanofi, Teva Pharma. J. Pelletier reports no disclosures. E. Thouvenot reports consulting and lecturing fees, travel grants, or unconditional research support from the following pharmaceutical companies: Actelion, Biogen, BMS, Merck, Novartis, and Roche. O. Heinzlef reports consulting and lecturing fees from Bayer Schering, Merck, Teva, Genzyme, Novartis, Almirall, and BiogenIdec; travel grants from Novartis, Teva, Genzyme, Merck Serono, and Biogen Idec; and research support from Roche, Merck, and Novartis. J.-P. Camdessanché received personal fees for lectures, consulting, writing of articles, or training courses from Akcea, Alexion, Alnylam, Argenx, Biogen, CSL Behring, Genzyme, Laboratoire Français des Biotechnologies (LFB), Merck, Novartis, Pfizer, Pharmalliance, Teva, Editions Scientifiques L&C, Edimark, Expression Santé, Natus, Scien, SNF-Floerger, not related to the submitted study, and he holds a patent on anti-FGFR3 autoantibodies and AGO autoantibodies in patients with sensory neuropathy. M. Fauvernier reports no disclosures. L. Remontet reports no disclosures. E. Leray reports consulting and lecture fees or travel grants from Biogen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, and Roche. Nothing related to the contents of the present work. Go to
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
