Real-World Investigation of Eosinophilic-Associated Disease Overlap (REVEAL): Analysis of a US Claims Database
- PMID: 37827978
- PMCID: PMC10570778
- DOI: 10.4168/aair.2023.15.5.580
Real-World Investigation of Eosinophilic-Associated Disease Overlap (REVEAL): Analysis of a US Claims Database
Abstract
Purpose: The epidemiology of eosinophil-associated diseases (EADs) is not yet fully understood. While some studies have been conducted on stand-alone eosinophilic diseases, there is scarce evidence on the degree of overlap among rarer conditions.
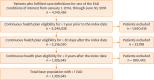
Methods: The retrospective Real-world inVestigation of Eosinophilic-Associated disease overLap (REVEAL) study used data from the Optum® Clinformatics® insurance claims database to describe and characterize disease overlap among 11 EADs: allergic bronchopulmonary aspergillosis, atopic dermatitis, chronic rhinosinusitis with nasal polyps, eosinophilic gastritis/gastroenteritis, eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis, bullous pemphigoid, chronic obstructive pulmonary disorder, chronic spontaneous urticaria, and non-cystic fibrosis bronchiectasis. Patient records with EADs of interest were identified between January 1, 2015, and June 30, 2018.
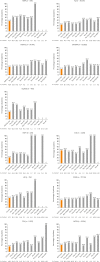
Results: Overall, 1,326,645 patients were included; 74.4% had 1 EAD, 20.5% had ≥ 2 EADs, and 5.1% had ≥ 3 EADs. Higher rates of disease overlap were associated with older age. Higher blood eosinophil counts were also observed in patients with a greater number of overlapping conditions, suggesting a common role for eosinophilic inflammation in the pathogenesis of multiple diseases. Furthermore, greater disease overlap was associated with higher disease severity in most cohorts.
Conclusions: Results from this study have implications for quantifying unmet needs and can be used to inform treatment guidelines and raise the awareness of eosinophilic inflammation and EAD overlap among healthcare professionals from a range of disease specialties.
Keywords: Inflammation; disease attributes; eosinophilia; eosinophils; epidemiology; multimorbidity.
Copyright © 2023 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
Anamaria Brailean, Justin Kwiatek, Danuta Kielar, Rohit Katial, Xia Wang, Xiao Xu, and Heide A. Stirnadel-Farrant are or were employees of AstraZeneca at the time of study conduct. Michael Stokes and Yong Jin Kim are or were employees of Evidera at the time of study conduct. Justin Kwiatek is a shareholder in AstraZeneca. Evidera received research funding from AstraZeneca for the design and implementation of analyses.
Figures



References
-
- Ruffner MA, Cianferoni A. Phenotypes and endotypes in eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2020;124:233–239. - PubMed
-
- Tsikrika S, Dimakou K, Papaioannou AI, Hillas G, Thanos L, Kostikas K, et al. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine. 2017;99:281–286. - PubMed

