An integrated personalized assistive devices approach to reduce the risk of foot ulcer recurrence in diabetes (DIASSIST): study protocol for a multicenter randomized controlled trial
- PMID: 37828618
- PMCID: PMC10568814
- DOI: 10.1186/s13063-023-07635-z
An integrated personalized assistive devices approach to reduce the risk of foot ulcer recurrence in diabetes (DIASSIST): study protocol for a multicenter randomized controlled trial
Abstract
Background: Preventing foot ulcers in people with diabetes can increase quality of life and reduce costs. Despite the availability of various interventions to prevent foot ulcers, recurrence rates remain high. We hypothesize that a multimodal treatment approach incorporating various footwear, self-management, and education interventions that matches an individual person's needs can reduce the risk of ulcer recurrence with beneficial cost-utility. The aim of this study is to assess the effect on foot ulcer recurrence, footwear adherence, and cost-utility of an integrated personalized assistive devices approach in high-risk people with diabetes.
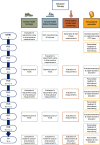
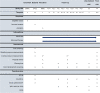
Methods: In a parallel-group multicenter randomized controlled trial, 126 adult participants with diabetes mellitus type 1 or 2, loss of protective sensation based on the presence of peripheral neuropathy, a healed plantar foot ulcer in the preceding 4 years, and possession of any type of custom-made footwear will be included. Participants will be randomly assigned to either enhanced therapy or usual care. Enhanced therapy consists of usual care and additionally a personalized treatment approach including pressure-optimized custom-made footwear, pressure-optimized custom-made footwear for indoor use, at-home daily foot temperature monitoring, and structured education, which includes motivational interviewing and personalized feedback on adherence and self-care. Participants will be followed for 12 months. Assessments include barefoot and in-shoe plantar pressure measurements; questionnaires concerning quality of life, costs, disease, and self-care knowledge; physical activity and footwear use monitoring; and clinical monitoring for foot ulcer outcomes. The study is powered for 3 primary outcomes: foot ulcer recurrence, footwear adherence, and cost-utility, the primary clinical, patient-related, and health-economic outcome respectively.
Discussion: This is the first study to integrate multiple interventions for ulcer prevention into a personalized state-of-the-art treatment approach and assess their combined efficacy in a randomized controlled trial in people with diabetes at high ulcer risk. Proven effectiveness, usability, and cost-utility will facilitate implementation in healthcare, improve the quality of life of high-risk people with diabetes, and reduce treatment costs.
Trial registration: ClinicalTrials.gov NCT05236660. Registered on 11 February 2022.
Keywords: Adherence; Assistive devices; Cost–benefit analysis; Diabetic foot ulcer; Education; Footwear; Motivational interviewing; Personalized medicine; Prevention; Temperature monitoring.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The cost-effectiveness and cost-utility of at-home infrared temperature monitoring in reducing the incidence of foot ulcer recurrence in patients with diabetes (DIATEMP): study protocol for a randomized controlled trial.Trials. 2018 Sep 24;19(1):520. doi: 10.1186/s13063-018-2890-2. Trials. 2018. PMID: 30249296 Free PMC article.
-
Effects of novel diabetic therapeutic footwear on preventing ulcer recurrence in patients with a history of diabetic foot ulceration: study protocol for an open-label, randomized, controlled trial.Trials. 2021 Feb 17;22(1):151. doi: 10.1186/s13063-021-05098-8. Trials. 2021. PMID: 33597005 Free PMC article.
-
Using motivational interviewing combined with digital shoe-fitting to improve adherence to wearing orthopedic shoes in people with diabetes at risk of foot ulceration: study protocol for a cluster-randomized controlled trial.Trials. 2021 Oct 28;22(1):750. doi: 10.1186/s13063-021-05680-0. Trials. 2021. PMID: 34711263 Free PMC article.
-
Risk assessments and structured care interventions for prevention of foot ulceration in diabetes: development and validation of a prognostic model.Health Technol Assess. 2020 Nov;24(62):1-198. doi: 10.3310/hta24620. Health Technol Assess. 2020. PMID: 33236718 Free PMC article. Review.
-
Footwear and insole design features that reduce neuropathic plantar forefoot ulcer risk in people with diabetes: a systematic literature review.J Foot Ankle Res. 2020 Jun 4;13(1):30. doi: 10.1186/s13047-020-00400-4. J Foot Ankle Res. 2020. PMID: 32498719 Free PMC article.
Cited by
-
Short-Term Efficacy of a Multi-Modal Intervention Program to Improve Custom-Made Footwear Use in People at High Risk of Diabetes-Related Foot Ulceration.J Clin Med. 2025 May 22;14(11):3635. doi: 10.3390/jcm14113635. J Clin Med. 2025. PMID: 40507403 Free PMC article.
References
-
- Time to Act: diabetes and foot care. A joint publication of the International Diabetes Federation and the International Working Group on the Diabetic Foot. 2005. https://www.worlddiabetesfoundation.org/files/diabetes-and-foot-care-tim.... Accessed 13 May 2022.
-
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. - PubMed
-
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377–384. - PubMed
-
- Lazzarini PA, Pacella RE, Armstrong DG, van Netten JJ. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet Med. 2018;35:1297–9. - PubMed
-
- Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia. 2005;48(9):1906–1910. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

