Antibodies to coagulase of Staphylococcus aureus crossreact to Efb and reveal different binding of shared fibrinogen binding repeats
- PMID: 37828992
- PMCID: PMC10565355
- DOI: 10.3389/fimmu.2023.1221108
Antibodies to coagulase of Staphylococcus aureus crossreact to Efb and reveal different binding of shared fibrinogen binding repeats
Abstract
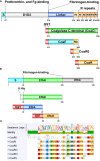
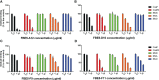
Staphylococcus aureus pathology is caused by a plethora of virulence factors able to combat multiple host defence mechanisms. Fibrinogen (Fg), a critical component in the host coagulation cascade, plays an important role in the pathogenesis of this bacterium, as it is the target of numerous staphylococcal virulence proteins. Amongst its secreted virulence factors, coagulase (Coa) and Extracellular fibrinogen-binding protein (Efb) share common Fg binding motives and have been described to form a Fg shield around staphylococcal cells, thereby allowing efficient bacterial spreading, phagocytosis escape and evasion of host immune system responses. Targeting these proteins with monoclonal antibodies thus represents a new therapeutic option against S. aureus. To this end, here we report the selection and characterization of fully human, sequence-defined, monoclonal antibodies selected against the C-terminal of coagulase. Given the functional homology between Coa and Efb, we also investigated if the generated antibodies bound the two virulence factors. Thirteen unique antibodies were isolated from naïve antibodies gene libraries by antibody phage display. As anticipated, most of the selected antibodies showed cross-recognition of these two proteins and among them, four were able to block the interaction between Coa/Efb and Fg. Furthermore, our monoclonal antibodies could interact with the two main Fg binding repeats present at the C-terminal of Coa and distinguish them, suggesting the presence of two functionally different Fg-binding epitopes.
Keywords: Efb; Staphylococcus aureus; coagulase; fibrinogen-binding repeats; monoclonal antibodies; phage display.
Copyright © 2023 Bertoglio, Ko, Thomas, Giordano, Scommegna, Meier, Polten, Becker, Arora, Hust, Höök and Visai.
Conflict of interest statement
Findings of this manuscript are part of patent application US 2020/0283508 on which FB, YPK, DM, MHu, MHö and LV are listed as inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Coagulase and Efb of Staphylococcus aureus Have a Common Fibrinogen Binding Motif.mBio. 2016 Jan 5;7(1):e01885-15. doi: 10.1128/mBio.01885-15. mBio. 2016. PMID: 26733070 Free PMC article.
-
The Complex Fibrinogen Interactions of the Staphylococcus aureus Coagulases.Front Cell Infect Microbiol. 2019 Apr 16;9:106. doi: 10.3389/fcimb.2019.00106. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31041195 Free PMC article.
-
Role for the fibrinogen-binding proteins coagulase and Efb in the Staphylococcus aureus-Candida interaction.Int J Med Microbiol. 2013 Jul;303(5):230-8. doi: 10.1016/j.ijmm.2013.02.011. Epub 2013 Mar 13. Int J Med Microbiol. 2013. PMID: 23684234
-
Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections.J Innate Immun. 2012;4(2):141-8. doi: 10.1159/000333447. Epub 2012 Jan 3. J Innate Immun. 2012. PMID: 22222316 Free PMC article. Review.
-
Staphylococcus aureus interactions with the endothelium: the role of bacterial "secretable expanded repertoire adhesive molecules" (SERAM) in disturbing host defense systems.Thromb Haemost. 2005 Aug;94(2):278-85. doi: 10.1160/TH05-05-0306. Thromb Haemost. 2005. PMID: 16113816 Review.
Cited by
-
Staphylococcus aureus Endocarditis Immunothrombosis.Metabolites. 2025 May 15;15(5):328. doi: 10.3390/metabo15050328. Metabolites. 2025. PMID: 40422904 Free PMC article. Review.
-
CAUTIon - not all UTIs are the same.Nat Rev Urol. 2025 Aug 1:10.1038/s41585-025-01065-z. doi: 10.1038/s41585-025-01065-z. Online ahead of print. Nat Rev Urol. 2025. PMID: 40751081 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

