Antibodies to coagulase of Staphylococcus aureus crossreact to Efb and reveal different binding of shared fibrinogen binding repeats
- PMID: 37828992
- PMCID: PMC10565355
- DOI: 10.3389/fimmu.2023.1221108
Antibodies to coagulase of Staphylococcus aureus crossreact to Efb and reveal different binding of shared fibrinogen binding repeats
Abstract
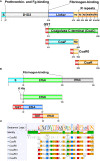
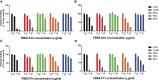
Staphylococcus aureus pathology is caused by a plethora of virulence factors able to combat multiple host defence mechanisms. Fibrinogen (Fg), a critical component in the host coagulation cascade, plays an important role in the pathogenesis of this bacterium, as it is the target of numerous staphylococcal virulence proteins. Amongst its secreted virulence factors, coagulase (Coa) and Extracellular fibrinogen-binding protein (Efb) share common Fg binding motives and have been described to form a Fg shield around staphylococcal cells, thereby allowing efficient bacterial spreading, phagocytosis escape and evasion of host immune system responses. Targeting these proteins with monoclonal antibodies thus represents a new therapeutic option against S. aureus. To this end, here we report the selection and characterization of fully human, sequence-defined, monoclonal antibodies selected against the C-terminal of coagulase. Given the functional homology between Coa and Efb, we also investigated if the generated antibodies bound the two virulence factors. Thirteen unique antibodies were isolated from naïve antibodies gene libraries by antibody phage display. As anticipated, most of the selected antibodies showed cross-recognition of these two proteins and among them, four were able to block the interaction between Coa/Efb and Fg. Furthermore, our monoclonal antibodies could interact with the two main Fg binding repeats present at the C-terminal of Coa and distinguish them, suggesting the presence of two functionally different Fg-binding epitopes.
Keywords: Efb; Staphylococcus aureus; coagulase; fibrinogen-binding repeats; monoclonal antibodies; phage display.
Copyright © 2023 Bertoglio, Ko, Thomas, Giordano, Scommegna, Meier, Polten, Becker, Arora, Hust, Höök and Visai.
Conflict of interest statement
Findings of this manuscript are part of patent application US 2020/0283508 on which FB, YPK, DM, MHu, MHö and LV are listed as inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

