Acid-base responsive multifunctional poly(formyl sulfide)s through a facile catalyst-free click polymerization of aldehyde-activated internal diynes and dithiols
- PMID: 37829011
- PMCID: PMC10566499
- DOI: 10.1039/d3sc03732k
Acid-base responsive multifunctional poly(formyl sulfide)s through a facile catalyst-free click polymerization of aldehyde-activated internal diynes and dithiols
Abstract
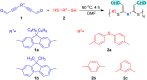
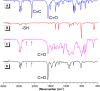
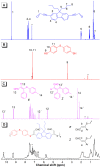
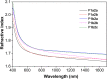
Acid-base equilibria play a critical role in biological processes and environmental systems. The development of innovative fluorescent polymeric materials to monitor acid-base equilibria is highly desirable. Herein, a novel catalyst-free click polymerization of aldehyde-activated internal diynes and dithiols was established, and exclusively Markovnikov poly(formyl sulfide)s (PFSs) with high molecular weights and moderate stereoregularity were produced in high yields. Because of the aromatic units and sulfur atoms in their main chains, these polymers possessed high refractive index values. By introducing the fluorene and aldehyde moieties, the resulting PFSs could act as a fluorescent sensor for sensitive hydrazine detection. Taking advantage of the reaction of the aldehyde group and hydrazine, imino-PFSs with remarkable and reversible fluorescence change through alternating fumigation with HCl and NH3 were easily acquired and further applied in multicolor patterning, a rewritable material and quadruple-mode information encryption. Additionally, a test strip of protonated imino-polymer for the tracking of bioamines in situ generated from marine product spoilage was also demonstrated. Collectively, this work not only provides a powerful click polymerization to enrich the multiplicity of sulfur-containing materials, but also opens up enormous opportunities for these functional polysulfides in diverse applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Hamilton D. G. Whiting M. J. Pryke S. R. Behav. Ecol. 2013;24:1138–1149. doi: 10.1093/beheco/art041. - DOI
LinkOut - more resources
Full Text Sources

