Post-synthetic benzylation of the mRNA 5' cap via enzymatic cascade reactions
- PMID: 37829022
- PMCID: PMC10566477
- DOI: 10.1039/d3sc03822j
Post-synthetic benzylation of the mRNA 5' cap via enzymatic cascade reactions
Abstract
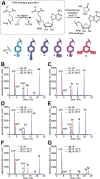
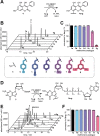
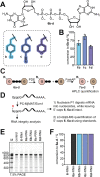
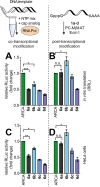
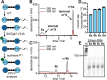
mRNAs are emerging modalities for vaccination and protein replacement therapy. Increasing the amount of protein produced by stabilizing the transcript or enhancing translation without eliciting a strong immune response are major steps towards overcoming the present limitations and improving their therapeutic potential. The 5' cap is a hallmark of mRNAs and non-natural modifications can alter the properties of the entire transcript selectively. Here, we developed a versatile enzymatic cascade for regioselective benzylation of various biomolecules and applied it for post-synthetic modification of mRNA at the 5' cap to demonstrate its potential. Starting from six synthetic methionine analogues bearing (hetero-)benzyl groups, S-adenosyl-l-methionine analogues are formed and utilized for N7G-cap modification of mRNAs. This post-synthetic enzymatic modification exclusively modifies mRNAs at the terminal N7G, producing mRNAs with functional 5' caps. It avoids the wrong orientation of the 5' cap-a problem in common co-transcriptional capping. In the case of the 4-chlorobenzyl group, protein production was increased to 139% during in vitro translation and to 128-150% in four different cell lines. This 5' cap modification did not activate cytosolic pathogen recognition receptors TLR3, TLR7 or TLR8 significantly more than control mRNAs, underlining its potential to contribute to the development of future mRNA therapeutics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Jackson L. A. Anderson E. J. Rouphael N. G. Roberts P. C. Makhene M. Coler R. N. McCullough M. P. Chappell J. D. Denison M. R. Stevens L. J. Pruijssers A. J. McDermott A. Flach B. Doria-Rose N. A. Corbett K. S. Morabito K. M. O'Dell S. Schmidt S. D. Swanson 2nd P. A. Padilla M. Mascola J. R. Neuzil K. M. Bennett H. Sun W. Peters E. Makowski M. Albert J. Cross K. Buchanan W. Pikaart-Tautges R. Ledgerwood J. E. Graham B. S. Beigel J. H. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. - DOI - PMC - PubMed
-
- Ramaswamy S. Tonnu N. Tachikawa K. Limphong P. Vega J. B. Karmali P. P. Chivukula P. Verma I. M. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E1941–e1950. doi: 10.1073/pnas.1619653114. - DOI - PMC - PubMed
- Perez-Garcia C. G. Diaz-Trelles R. Vega J. B. Bao Y. Sablad M. Limphong P. Chikamatsu S. Yu H. Taylor W. Karmali P. P. Tachikawa K. Chivukula P. Mol. Ther.--Nucleic Acids. 2022;28:87–98. doi: 10.1016/j.omtn.2022.02.020. - DOI - PMC - PubMed
-
- Strzelecka D. Smietanski M. Sikorski P. J. Warminski M. Kowalska J. Jemielity J. RNA. 2020;26:1815–1837. doi: 10.1261/rna.077099.120. - DOI - PMC - PubMed
- Wojtczak B. A. Sikorski P. J. Fac-Dabrowska K. Nowicka A. Warminski M. Kubacka D. Nowak E. Nowotny M. Kowalska J. Jemielity J. J. Am. Chem. Soc. 2018;140:5987–5999. doi: 10.1021/jacs.8b02597. - DOI - PubMed
- Drazkowska K. Tomecki R. Warminski M. Baran N. Cysewski D. Depaix A. Kasprzyk R. Kowalska J. Jemielity J. Sikorski P. J. Nucleic Acids Res. 2022;50:9051–9071. doi: 10.1093/nar/gkac722. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

