Rh(iii)-catalyzed highly site- and regio-selective alkenyl C-H activation/annulation of 4-amino-2-quinolones with alkynes via reversible alkyne insertion
- PMID: 37829027
- PMCID: PMC10566469
- DOI: 10.1039/d3sc03987k
Rh(iii)-catalyzed highly site- and regio-selective alkenyl C-H activation/annulation of 4-amino-2-quinolones with alkynes via reversible alkyne insertion
Abstract
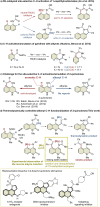
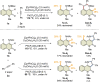
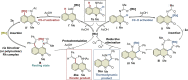
3,4-Fused 2-quinolone frameworks are important structural motifs found in natural products and biologically active compounds. Intermolecular alkenyl C-H activation/annulation of 4-amino-2-quinolone substrates with alkynes is one of the most efficient methods for accessing such structural motifs. However, this is a formidable challenge because 4-amino-2-quinolones have two cleavable C-H bonds: an alkenyl C-H bond at the C3-position and an aromatic C-H bond at the C5-position. Herein, we report the Rh(iii)-catalyzed highly site-selective alkenyl C-H functionalization of 4-amino-2-quinolones to afford 3,4-fused 2-quinolones. This method has a wide substrate scope, including unsymmetrical internal alkynes, with complete regioselectivity. Several control experiments using an isolated key intermediate analog suggested that the annulation reaction proceeds via reversible alkyne insertion involving a binuclear Rh complex although alkyne insertion is generally recognized as an irreversible process due to the high activation barrier of the reverse process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
-
For recent reviews, see:
- Zhu R. Farmer M. E. Chen Y. Yu J. Angew. Chem., Int. Ed. 2016;55:10578–10599. doi: 10.1002/anie.201600791. - DOI - PMC - PubMed
- Brandhofer T. García Mancheño O. Eur. J. Org Chem. 2018;2018:6050–6067. doi: 10.1002/ejoc.201800896. - DOI
- Baccalini A. Faita G. Zanoni G. Maiti D. Chem. - Eur. J. 2020;26:9749–9783. doi: 10.1002/chem.202001832. - DOI - PubMed
- Baudoin O. Angew. Chem., Int. Ed. 2020;59:17798–17809. doi: 10.1002/anie.202001224. - DOI - PubMed
- Zhang Y. Szostak M. Chem. - Eur. J. 2022;28:e202104278. doi: 10.1002/chem.202104278. - DOI - PMC - PubMed
-
-
-
For selected reviews, see:
- He R. Huang Z.-T. Zheng Q.-Y. Wang C. Tetrahedron Lett. 2014;55:5705–5713. doi: 10.1016/j.tetlet.2014.08.077. - DOI
- Cui X. Mo J. Wang L. Liu Y. Synthesis. 2015;47:439–459. doi: 10.1055/s-0034-1379890. - DOI
- Gandeepan P. Cheng C.-H. Chem.–Asian J. 2016;11:448–460. doi: 10.1002/asia.201501186. - DOI - PubMed
- Yang Y. Li K. Cheng Y. Wan D. Li M. You J. Chem. Commun. 2016;52:2872–2884. doi: 10.1039/C5CC09180B. - DOI - PubMed
- Peneau A. Guillou C. Chabaud L. Eur. J. Org Chem. 2018;2018:5777–5794. doi: 10.1002/ejoc.201800298. - DOI - PubMed
- Wang C. Chen F. Qian P. Cheng J. Org. Biomol. Chem. 2021;19:1705–1721. doi: 10.1039/D0OB02377A. - DOI - PubMed
- Saha A. Shankar M. Sau S. Sahoo A. K. Chem. Commun. 2022;58:4561–4587. doi: 10.1039/D2CC00172A. - DOI - PubMed
-
-
- Martínez Á. M. Echavarren J. Alonso I. Rodríguez N. Arrayás R. G. Carretero J. C. Chem. Sci. 2015;6:5802–5814. doi: 10.1039/C5SC01885D. - DOI - PMC - PubMed
- Elumalai K. Leong W. K. Tetrahedron Lett. 2018;59:113. doi: 10.1016/j.tetlet.2017.11.058. - DOI
- Pabst T. P. Obligacion J. V. Rochette É. Pappas I. Chirik P. J. J. Am. Chem. Soc. 2019;141:15378–15389. doi: 10.1021/jacs.9b07984. - DOI - PMC - PubMed
- Alharis R. A. Mcmullin C. L. Davies D. L. Singh K. Macgregor S. A. J. Am. Chem. Soc. 2019;141:8896–8906. doi: 10.1021/jacs.9b02073. - DOI - PubMed
- Prim D. Large B. Synthesis. 2020;52:2600–2612. doi: 10.1055/s-0040-1707855. - DOI
-
- Bedford R. B. Durrant S. J. Montgomery M. Angew. Chem., Int. Ed. 2015;54:8787–8790. doi: 10.1002/anie.201502150. - DOI - PMC - PubMed
- Leitch J. A. Bhonoah Y. Frost C. G. ACS Catal. 2017;7:5618–5627. doi: 10.1021/acscatal.7b01785. - DOI
- Kang D. Ahn K. Hong S. Asian J. Org. Chem. 2018;7:1136–1150. doi: 10.1002/ajoc.201800128. - DOI
- Biswas A. Maity S. Pan S. Samanta R. Chem.–Asian J. 2020;15:2092–2109. doi: 10.1002/asia.202000506. - DOI - PubMed
- Corio A. Gravier-Pelletier C. Busca P. Molecules. 2021;26:5467. doi: 10.3390/molecules26185467. - DOI - PMC - PubMed
- Yamamoto Y. in Handbook of CH-Functionalization, ed. D. Maiti, Wiley, 2022
LinkOut - more resources
Full Text Sources
Miscellaneous

