Ni-Catalyzed Regio- and Stereoselective Alkylarylation of Unactivated Alkenes in γ,δ-Alkenylketimines
- PMID: 37829145
- PMCID: PMC10569404
- DOI: 10.1021/acscatal.2c01697
Ni-Catalyzed Regio- and Stereoselective Alkylarylation of Unactivated Alkenes in γ,δ-Alkenylketimines
Abstract
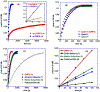
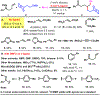
We disclose a Ni-catalyzed vicinal alkylarylation of unactivated alkenes in γ,δ-alkenylketimines with aryl halides and alkylzinc reagents. The reaction produces γ-C(sp3)-branched δ-arylketones with the construction of two new C(sp3)-C(sp3) and C(sp3)-C(sp2) bonds. Electron-deficient alkenes play crucial dual roles as ligands to stabilize reaction intermediates and to increase catalytic rates for the formation of C(sp3)-C(sp3) bonds. This alkene alkylarylation reaction is also effective for secondary alkylzinc reagents and internal alkenes, and proceeds with a complete regio- and stereocontrol, affording products with up to three contiguous all-carbon all-cis secondary stereocenters.
Keywords: C(sp3)-C(sp3); alkenes; alkylarylation; imines; nickel-catalyzed; stereoselective.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

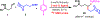
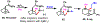
References
-
- For reviews, see:Dhungana RK; KC S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314–1340; - PubMed
- Giri R; Kc S Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem 2018, 83, 3013–3022; - PubMed
- Derosa J; Apolinar O; Kang T; Tran VT; Engle KM Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes. Chem. Sci 2020, 11, 4287–4296; - PMC - PubMed
- Badir SO; Molander GA Developments in Photoredox/Nickel Dual-Catalyzed 1,2-Difunctionalizations. Chem 2020, 6, 1327–1339; - PMC - PubMed
- Guo L; Yuan M; Zhang Y; Wang F; Zhu S; Gutierrez O; Chu L General Method for Enantioselective Three-Component Carboarylation of Alkenes Enabled by Visible-Light Dual Photoredox/Nickel Catalysis. J. Am. Chem. Soc 2020, 142, 20390–20399; - PMC - PubMed
- Tu H-Y; Zhu S; Qing F-L; Chu L Recent Advances in Nickel-Catalyzed Three-Component Difunctionalization of Unactivated Alkenes. Synthesis 2020, 52, 1346–1356;
- Montgomery J Nickel-Catalyzed Cyclizations, Couplings, and Cycloadditions Involving Three Reactive Components. Acc. Chem. Res 2000, 33, 467–473. - PubMed
-
- Qin T; Cornella J; Li C; Malins LR; Edwards JT; Kawamura S; Maxwell BD; Eastgate MD; Baran PS A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805; - PMC - PubMed
- Zhang L; Lovinger GJ; Edelstein EK; Szymaniak AA; Chierchia MP; Morken JP Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement. Science 2016, 351, 70–74. - PMC - PubMed
-
- Gao P; Chen L-A; Brown MK Nickel-Catalyzed Stereoselective Diarylation of Alkenylarenes. J. Am. Chem. Soc 2018, 140, 10653–10657; - PMC - PubMed
- Shrestha B; Basnet P; Dhungana RK; Kc S; Thapa S; Sears JM; Giri R Ni-Catalyzed Regioselective 1,2-Dicarbofunctionalization of Olefins by Intercepting Heck Intermediates as Imine-Stabilized Transient Metallacycles. J. Am. Chem. Soc 2017, 139, 10653–10656; - PubMed
- Thapa S; Dhungana RK; Magar RT; Shrestha B; Kc S; Giri R Ni-catalysed regioselective 1,2-diarylation of unactivated olefins by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles. Chem. Sci 2018, 9, 904–909; - PMC - PubMed
- Anthony D; Lin Q; Baudet J; Diao T Nickel-Catalyzed Asymmetric Reductive Diarylation of Vinylarenes. Angew. Chem. Int. Ed 2019, 58, 3198–3202; - PMC - PubMed
- Basnet P; Kc S; Dhungana RK; Shrestha B; Boyle TJ; Giri R Synergistic Bimetallic Ni/Ag and Ni/Cu Catalysis for Regioselective γ,δ-Diarylation of Alkenyl Ketimines: Addressing β-H Elimination by in Situ Generation of Cationic Ni(II) Catalysts. J. Am. Chem. Soc 2018, 140, 15586–15590; - PubMed
- Basnet P; Dhungana RK; Thapa S; Shrestha B; Kc S; Sears JM; Giri R Ni-Catalyzed Regioselective β,δ-Diarylation of Unactivated Olefins in Ketimines via Ligand-Enabled Contraction of Transient Nickellacycles: Rapid Access to Remotely Diarylated Ketones. J. Am. Chem. Soc 2018, 140, 7782–7786; - PubMed
- Derosa J; Kleinmans R; Tran VT; Karunananda MK; Wisniewski SR; Eastgate MD; Engle KM Nickel-Catalyzed 1,2-Diarylation of Simple Alkenyl Amides. J. Am. Chem. Soc 2018, 140, 17878–17883; - PubMed
- Kleinmans R; Apolinar O; Derosa J; Karunananda MK; Li Z-Q; Tran VT; Wisniewski SR; Engle KM Ni-Catalyzed 1,2-Diarylation of Alkenyl Ketones: A Comparative Study of Carbonyl-Directed Reaction Systems. Org. Lett 2021, 23, 5311–5316; - PubMed
- Urkalan KB; Sigman MS Palladium-Catalyzed Oxidative Intermolecular Difunctionalization of Terminal Alkenes with Organostannanes and Molecular Oxygen. Angew. Chem. Int. Ed 2009, 48, 3146–3149; - PMC - PubMed
- Ouyang X-H; Cheng J; Li J-H 1,2-Diarylation of alkenes with aryldiazonium salts and arenes enabled by visible light photoredox catalysis. Chem. Commun 2018, 54, 8745–8748; - PubMed
- Zhang Y; Chen G; Zhao D Three-component vicinal-diarylation of alkenes via direct transmetalation of arylboronic acids. Chem. Sci 2019, 10, 7952–7957; - PMC - PubMed
- Derosa J; Kang T; Tran VT; Wisniewski SR; Karunananda MK; Jankins TC; Xu KL; Engle KM Nickel-Catalyzed 1,2-Diarylation of Alkenyl Carboxylates: A Gateway to 1,2,3-Trifunctionalized Building Blocks. Angew. Chem. Int. Ed 2020, 59, 1201–1205; - PubMed
- Jeon J; Ryu H; Lee C; Cho D; Baik M-H; Hong S Site-Selective 1,1-Difunctionalization of Unactivated Alkenes Enabled by Cationic Palladium Catalysis. J. Am. Chem. Soc 2019, 141, 10048–10059; - PubMed
- Li Z; Wu D; Ding C; Yin G Modular Synthesis of Diarylalkanes by Nickel-Catalyzed 1,1-Diarylation of Unactivated Terminal Alkenes. CCS Chemistry 2020, 2, 576–582;
- Myhill JA; Wilhelmsen CA; Zhang L; Morken JP Diastereoselective and Enantioselective Conjunctive Cross-Coupling Enabled by Boron Ligand Design. J. Am. Chem. Soc 2018, 140, 15181–15185; - PMC - PubMed
- Lv H; Xiao L-J; Zhao D; Zhou Q-L Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. Chem. Sci 2018, 9, 6839–6843. - PMC - PubMed
-
- Dhungana RK; Kc S; Basnet P; Aryal V; Chesley LJ; Giri R Ni(I)-Catalyzed β,δ-Vinylarylation of γ,δ-Alkenyl α-Cyanocarboxylic Esters via Contraction of Transient Nickellacycles. ACS Catal. 2019, 9, 10887–10893; - PMC - PubMed
- Kuang Z; Yang K; Song Q Pd-Catalyzed Regioselective 1,2-Difunctionalization of Vinylarenes with Alkenyl Triflates and Aryl Boronic Acids at Ambient Temperature. Org. Lett 2017, 19, 2702–2705. - PubMed
-
- Li W; Boon JK; Zhao Y Nickel-catalyzed difunctionalization of allyl moieties using organoboronic acids and halides with divergent regioselectivities. Chem. Sci 2018, 9, 600–607; - PMC - PubMed
- Pan R; Shi C; Zhang D; Tian Y; Guo S; Yao H; Lin A Nickel-Catalyzed Reductive 1,2-Dialkynylation of Alkenes Bearing an 8-Aminoquinoline Directing Group. Org. Lett 2019, 21, 8915–8920. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
