Seven-Membered Cyclic Diamidoalumanyls of Heavier Alkali Metals: Structures and C-H Activation of Arenes
- PMID: 37829511
- PMCID: PMC10565898
- DOI: 10.1021/acs.organomet.3c00323
Seven-Membered Cyclic Diamidoalumanyls of Heavier Alkali Metals: Structures and C-H Activation of Arenes
Abstract
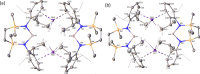
Like the previously reported potassium-based system, rubidium and cesium reduction of [{SiNDipp}AlI] ({SiNDipp} = {CH2SiMe2NDipp}2) with the heavier alkali metals [M = Rb and Cs] provides dimeric group 1 alumanyl derivatives, [{SiNDipp}AlM]2. In contrast, similar treatment with sodium results in over-reduction and incorporation of a formal equivalent of [{SiNDipp}Na2] into the resultant sodium alumanyl species. The dimeric K, Rb, and Cs compounds display a variable efficacy toward the C-H oxidative addition of arene C-H bonds at elevated temperatures (Cs > Rb > K, 110 °C) to yield (hydrido)(organo)aluminate species. Consistent with the synthetic experimental observations, computational (DFT) assessment of the benzene C-H activation indicates that rate-determining attack of the Al(I) nucleophile within the dimeric species is facilitated by π-engagement of the arene with the electrophilic M+ cation, which becomes increasingly favorable as group 1 is descended.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Terminal Alkyne Activation by an Al(I)-Centered Anion: Impact on the Mechanism of Alkali Metal Identity.Organometallics. 2024 Dec 9;44(1):236-243. doi: 10.1021/acs.organomet.4c00435. eCollection 2025 Jan 13. Organometallics. 2024. PMID: 39822185 Free PMC article.
-
An Alumanyl Test Case of Group 1 Redox Interchange.Chemistry. 2025 Aug 7;31(44):e202502197. doi: 10.1002/chem.202502197. Epub 2025 Jul 21. Chemistry. 2025. PMID: 40627841 Free PMC article.
-
Seven-Membered Cyclic Potassium Diamidoalumanyls.Chemistry. 2021 Oct 25;27(60):14971-14980. doi: 10.1002/chem.202102682. Epub 2021 Sep 21. Chemistry. 2021. PMID: 34403562 Free PMC article.
-
On the reactivity of Al-group 11 (Cu, Ag, Au) bonds.Dalton Trans. 2022 Mar 8;51(10):3913-3924. doi: 10.1039/d2dt00404f. Dalton Trans. 2022. PMID: 35169824
-
Calcium-Catalyzed Arene C-H Bond Activation by Low-Valent AlI.Angew Chem Int Ed Engl. 2019 Oct 21;58(43):15496-15503. doi: 10.1002/anie.201908978. Epub 2019 Sep 13. Angew Chem Int Ed Engl. 2019. PMID: 31465144 Free PMC article. Review.
Cited by
-
Terminal Alkyne Activation by an Al(I)-Centered Anion: Impact on the Mechanism of Alkali Metal Identity.Organometallics. 2024 Dec 9;44(1):236-243. doi: 10.1021/acs.organomet.4c00435. eCollection 2025 Jan 13. Organometallics. 2024. PMID: 39822185 Free PMC article.
-
Allosteric Differentiation of Al(I) Reactivity.Chemistry. 2025 May 27;31(30):e202501352. doi: 10.1002/chem.202501352. Epub 2025 Apr 25. Chemistry. 2025. PMID: 40211470 Free PMC article.
-
An Alumanyl Test Case of Group 1 Redox Interchange.Chemistry. 2025 Aug 7;31(44):e202502197. doi: 10.1002/chem.202502197. Epub 2025 Jul 21. Chemistry. 2025. PMID: 40627841 Free PMC article.
-
Application of Bis(amido)alkyl Magnesiates toward the Synthesis of Molecular Rubidium and Cesium Hydrido-magnesiates.Organometallics. 2024 Jun 13;43(12):1393-1401. doi: 10.1021/acs.organomet.4c00190. eCollection 2024 Jun 24. Organometallics. 2024. PMID: 38938897 Free PMC article.
-
Alumanyl Reduction, Reductive Coupling and C-H Isomerization of Organic Nitriles.Organometallics. 2024 Aug 16;43(17):1938-1945. doi: 10.1021/acs.organomet.4c00289. eCollection 2024 Sep 9. Organometallics. 2024. PMID: 39268183 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
