Uncovering key clinical trial features influencing recruitment
- PMID: 37830010
- PMCID: PMC10565197
- DOI: 10.1017/cts.2023.623
Uncovering key clinical trial features influencing recruitment
Abstract
Background: Randomized clinical trials (RCT) are the foundation for medical advances, but participant recruitment remains a persistent barrier to their success. This retrospective data analysis aims to (1) identify clinical trial features associated with successful participant recruitment measured by accrual percentage and (2) compare the characteristics of the RCTs by assessing the most and least successful recruitment, which are indicated by varying thresholds of accrual percentage such as ≥ 90% vs ≤ 10%, ≥ 80% vs ≤ 20%, and ≥ 70% vs ≤ 30%.
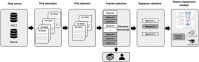
Methods: Data from the internal research registry at Columbia University Irving Medical Center and Aggregated Analysis of ClinicalTrials.gov were collected for 393 randomized interventional treatment studies closed to further enrollment. We compared two regularized linear regression and six tree-based machine learning models for accrual percentage (i.e., reported accrual to date divided by the target accrual) prediction. The outperforming model and Tree SHapley Additive exPlanations were used for feature importance analysis for participant recruitment. The identified features were compared between the two subgroups.
Results: CatBoost regressor outperformed the others. Key features positively associated with recruitment success, as measured by accrual percentage, include government funding and compensation. Meanwhile, cancer research and non-conventional recruitment methods (e.g., websites) are negatively associated with recruitment success. Statistically significant subgroup differences (corrected p-value < .05) were found in 15 of the top 30 most important features.
Conclusion: This multi-source retrospective study highlighted key features influencing RCT participant recruitment, offering actionable steps for improvement, including flexible recruitment infrastructure and appropriate participant compensation.
Keywords: Clinical trials; SHAP; informatics; machine learning; research recruitment.
© The Author(s) 2023.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Direct-to-Consumer Recruitment Methods via Traditional and Social Media to Aid in Research Accrual for Clinical Trials for Rare Diseases: Comparative Analysis Study.J Med Internet Res. 2023 Mar 14;25:e39262. doi: 10.2196/39262. J Med Internet Res. 2023. PMID: 36917158 Free PMC article.
-
Performance and predictors of recruitment success in National Heart, Lung, and Blood Institute's cardiovascular clinical trials.Clin Trials. 2018 Oct;15(5):444-451. doi: 10.1177/1740774518792271. Epub 2018 Aug 7. Clin Trials. 2018. PMID: 30084662
-
Publication Bias in Gastrointestinal Oncology Trials Performed over the Past Decade.Oncologist. 2021 Aug;26(8):660-667. doi: 10.1002/onco.13759. Epub 2021 Mar 31. Oncologist. 2021. PMID: 33728733 Free PMC article.
-
Interpretable machine learning model to predict surgical difficulty in laparoscopic resection for rectal cancer.Front Oncol. 2024 Feb 6;14:1337219. doi: 10.3389/fonc.2024.1337219. eCollection 2024. Front Oncol. 2024. PMID: 38380369 Free PMC article. Review.
-
Randomized controlled trials comparing surgery to non-operative management in neurosurgery: a systematic review.Acta Neurochir (Wien). 2019 Apr;161(4):627-634. doi: 10.1007/s00701-019-03849-w. Epub 2019 Feb 23. Acta Neurochir (Wien). 2019. PMID: 30798479 Free PMC article.
Cited by
-
Clinical researchers' insights on key data for eligibility screening in clinical studies.J Clin Transl Sci. 2024 Oct 16;8(1):e167. doi: 10.1017/cts.2024.617. eCollection 2024. J Clin Transl Sci. 2024. PMID: 39619069 Free PMC article.
-
Scientia pro bono humani generis: Science for the benefit of humanity.J Clin Transl Sci. 2024 Feb 20;8(1):e29. doi: 10.1017/cts.2023.696. eCollection 2024. J Clin Transl Sci. 2024. PMID: 38384907 Free PMC article. No abstract available.
-
A scoping review of artificial intelligence applications in clinical trial risk assessment.NPJ Digit Med. 2025 Jul 30;8(1):486. doi: 10.1038/s41746-025-01886-7. NPJ Digit Med. 2025. PMID: 40731070 Free PMC article.
-
CLEAR: A vision to support clinical evidence lifecycle with continuous learning.J Biomed Inform. 2025 Jul 29;169:104884. doi: 10.1016/j.jbi.2025.104884. Online ahead of print. J Biomed Inform. 2025. PMID: 40744316
-
Recruitment and Retention Strategies for Historically Marginalized Populations in Colorectal Cancer Trials: A Cross-Sectional Analysis Using Systematic Review Methods.J Gastrointest Cancer. 2024 Nov 26;56(1):24. doi: 10.1007/s12029-024-01146-z. J Gastrointest Cancer. 2024. PMID: 39586872 Free PMC article.
References
-
- US Food and Drug Administration. Global Participation in Clinical Trials Report 2015-2019, (https://www.fda.gov/media/143592/download?utm_medium=email&utm_source=go...). Accessed January 10, 2023.
Grants and funding
LinkOut - more resources
Full Text Sources
