A randomized, double-blind, placebo-controlled, parallel-group 12-week pilot phase II trial of SaiLuoTong (SLT) for cognitive function in older adults with mild cognitive impairment
- PMID: 37830013
- PMCID: PMC10565903
- DOI: 10.1002/trc2.12420
A randomized, double-blind, placebo-controlled, parallel-group 12-week pilot phase II trial of SaiLuoTong (SLT) for cognitive function in older adults with mild cognitive impairment
Abstract
Introduction: This study primarily aimed to evaluate the efficacy and safety of SaiLuoTong (SLT) on cognition in mild cognitive impairment (MCI).
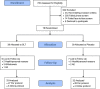
Methods: Community-dwelling people with MCI aged ≥60 years were randomly assigned to 180 mg/day SLT or placebo for 12 weeks.
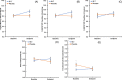
Results: Thirty-nine participants were randomized to each group (N = 78); 65 were included in the final analysis. After 12 weeks, the between-groups difference in Logical Memory delayed recall scores was 1.40 (95% confidence interval [CI]: 0.22 to 2.58; P = 0.010); Delis-Kaplan Executive Function System Trail Making Test Condition 4 switching and contrast scaled scores were 1.42 (95% CI: -0.15 to 2.99; P = 0.038) and 1.56 (95% CI: -0.09 to 3.20; P = 0.032), respectively; Rey Auditory Verbal Learning Test delayed recall was 1.37 (95% CI: -0.10 to 2.84; P = 0.034); and Functional Activities Questionnaire was 1.21 (95% CI: -0.21 to 2.63; P = 0.047; P < 0.001 after controlling for baseline scores).
Discussion: SLT is well tolerated and may be useful in supporting aspects of memory retrieval and executive function in people with MCI.
Highlights: SaiLuoTong (SLT) improves delayed memory retrieval and executive function in people with mild cognitive impairment (MCI).SLT is well tolerated in people ≥ 60 years.The sample of community dwellers with MCI was well characterized and homogeneous.
Keywords: Alzheimer's disease; SaiLuoTong; clinical trial; cognitive function; mild cognitive impairment.
© 2023 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
As a medical research institute, NICM Health Research Institute receives research grants and donations from foundations, universities, government agencies, individuals, and industry. Sponsors and donors provide untied funding for work to advance the vision and mission of the Institute. The project that is the subject of this article was not undertaken as part of a contractual relationship with any organisation other than the funding declared. Author disclosures are available in the supporting information.
Figures
References
-
- Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. - PMC - PubMed
-
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183‐194. - PubMed
-
- Brodaty H, Heffernan M, Kochan NA, et al. Mild cognitive impairment in a community sample: the Sydney memory and ageing study. Alzheimers Dement. 2013;9(3):310‐317.e1. - PubMed
LinkOut - more resources
Full Text Sources
