NAD+ Acts as a Protective Factor in Cellular Stress Response to DNA Alkylating Agents
- PMID: 37830610
- PMCID: PMC10572126
- DOI: 10.3390/cells12192396
NAD+ Acts as a Protective Factor in Cellular Stress Response to DNA Alkylating Agents
Abstract
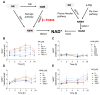
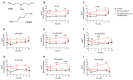
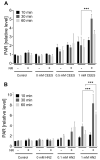
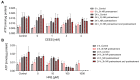
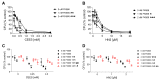
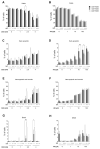
Sulfur mustard (SM) and its derivatives are potent genotoxic agents, which have been shown to trigger the activation of poly (ADP-ribose) polymerases (PARPs) and the depletion of their substrate, nicotinamide adenine dinucleotide (NAD+). NAD+ is an essential molecule involved in numerous cellular pathways, including genome integrity and DNA repair, and thus, NAD+ supplementation might be beneficial for mitigating mustard-induced (geno)toxicity. In this study, the role of NAD+ depletion and elevation in the genotoxic stress response to SM derivatives, i.e., the monofunctional agent 2-chloroethyl-ethyl sulfide (CEES) and the crosslinking agent mechlorethamine (HN2), was investigated with the use of NAD+ booster nicotinamide riboside (NR) and NAD+ synthesis inhibitor FK866. The effects were analyzed in immortalized human keratinocytes (HaCaT) or monocyte-like cell line THP-1. In HaCaT cells, NR supplementation, increased NAD+ levels, and elevated PAR response, however, did not affect ATP levels or DNA damage repair, nor did it attenuate long- and short-term cytotoxicities. On the other hand, the depletion of cellular NAD+ via FK866 sensitized HaCaT cells to genotoxic stress, particularly CEES exposure, whereas NR supplementation, by increasing cellular NAD+ levels, rescued the sensitizing FK866 effect. Intriguingly, in THP-1 cells, the NR-induced elevation of cellular NAD+ levels did attenuate toxicity of the mustard compounds, especially upon CEES exposure. Together, our results reveal that NAD+ is an important molecule in the pathomechanism of SM derivatives, exhibiting compound-specificity. Moreover, the cell line-dependent protective effects of NR are indicative of system-specificity of the application of this NAD+ booster.
Keywords: DNA damage; NAD booster; PARP; mustard agents; nicotinamide adenine dinucleotide; nicotinamide riboside; poly(ADP-ribosylation); sulfur mustard.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

