Synthesis of the A-F Fragment of the Pacific Ciguatoxin CTX3C by Iterative Ring-Closing Metathesis and Tsuji-Trost Allylation
- PMID: 37830907
- PMCID: PMC10946863
- DOI: 10.1002/chem.202303121
Synthesis of the A-F Fragment of the Pacific Ciguatoxin CTX3C by Iterative Ring-Closing Metathesis and Tsuji-Trost Allylation
Abstract
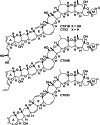
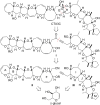
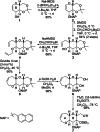
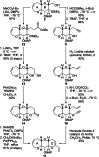
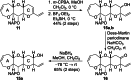
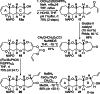
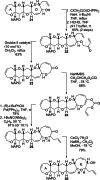
The fully functionalized A-F fragment of the Pacific ciguatoxin CTX3C has been synthesized from a derivative of D-glucal, which serves as the B-ring. Rings A and C were introduced to either side of ring B by ring-closing diene and enyne metathesis (RCM). The seven-membered D-ring and eight-membered E-ring were assembled by iterative use of a six-step reaction sequence in which RCM was used for ring construction and Tsuji-Trost allylation was employed for subsequent stereoselective functionalization. The nine-membered F-ring was formed by use of an RCM reaction and bears the functionality required for attachment of the I-M fragment and subsequent closure of rings G and H.
Keywords: Tsuji-Trost allylation; ciguatoxin; iterative ring construction; polyether; ring-closing metathesis.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
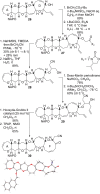
References
-
- None
-
- Murata M., Legrand A. M., Ishibashi Y., Yasumoto T., J. Am. Chem. Soc. 1989, 111, 8929–8931;
-
- Murata M., Legrand A. M., Ishibashi Y., Fukui M., Yasumoto T., J. Am. Chem. Soc. 1990, 112, 4380–4386;
-
- Satake M., Murata M., Yasumoto T., Tetrahedron Lett. 1993, 34, 1975–1978;
-
- Yasumoto T., Igarashi T., Legrand A.-M., Cruchet P., Chinain M., Fujita T., Naoki H., J. Am. Chem. Soc. 2000, 122, 4988–4989;
Grants and funding
- EP/R035423/1/Engineering and Physical Sciences Research Council
- EP/K503058/Engineering and Physical Sciences Research Council
- EP/L50497X/Engineering and Physical Sciences Research Council
- EP/M506539/Engineering and Physical Sciences Research Council
- EP/R513222/1/Engineering and Physical Sciences Research Council

